Podding of Paralomis Granulosa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blancpain Ocean Commitment Partnerships
Blancpain Ocean Commitment September 2020 The partnerships Blancpain Ocean Commitment Partnerships Exploration and preservation of the world’s oceans is core to Blancpain. With its almost 70-year legacy of the Fifty Fathoms diving watch, the Brand has woven close ties with the explorers, photographers, scientists, and environmentalists who treasure this precious resource. With that affinity has come a determination to support important activities and initiatives dedicated to the oceans. For the past several years, the Blancpain Ocean Commitment (BOC) has demonstrated consistent support for oceanographic initiatives and partnerships with leading organizations, such as the Pristine Seas expeditions, Laurent Ballesta’s Gombessa project, the World Ocean Initiative organized by The Economist, and the World Oceans Day, celebrated every year at the United Nations headquarters in New York. Blancpain was a frontrunner in backing the Pristine Seas initiative as founding partner from 2011 to 2016. Headed by National Geographic Society's Explorer-in-Residence, Dr. Enric Sala, the Pristine Seas expeditions were dedicated to exploring and protecting the precious few remaining, truly unspoiled, wild ocean areas. The expeditions studied and filmed these areas as part of the effort to educate the public and governments on the value and uniqueness of their ecosystems, and to secure governmental pledges as well as support from local communities to protect them. The program helped in particular to protect marine areas in the United States, Chile, Gabon, Kiribati, Costa Rica, French Polynesia, the Seychelles, northern Greenland, and South America's Patagonia region. Laurent Ballesta's Gombessa project focuses on studying some of the rarest, most elusive marine creatures and phenomena. -

Challenging the Cold: Crabs Reconquer the Antarctic
Ecology, 86(3), 2005, pp. 619±625 q 2005 by the Ecological Society of America CHALLENGING THE COLD: CRABS RECONQUER THE ANTARCTIC SVEN THATJE,1,5 KLAUS ANGER,2 JAVIER A. CALCAGNO,3 GUSTAVO A. LOVRICH,4 HANS-OTTO POÈ RTNER,1 AND WOLF E. ARNTZ1 1Alfred Wegener Institute for Polar and Marine Research, Columbusstr. D-27568 Bremerhaven, Germany 2Biologische Anstalt Helgoland, Foundation Alfred Wegener Institute, Helgoland, Germany 3Universidad de Buenos Aires, Facultad de Ciencias Exactas y Naturales, Intendente GuÈiraldes 2160, C1428EHA, Buenos Aires, Argentina 4Consejo Nacional de Investigaciones Cientõ®cas y TeÂcnicas, Centro Austral de Investigaciones Cientõ®cas, CC 92, V9410BFD Ushuaia, Tierra del Fuego, Argentina Abstract. Recent records of lithodid crabs in deeper waters off the Antarctic continental slope raised the question of the return of crabs to Antarctic waters, following their extinction in the lower Miocene ;15 million years ago. Antarctic cooling may be responsible for the impoverishment of the marine high Antarctic decapod fauna, presently comprising only ®ve benthic shrimp species. Effects of polar conditions on marine life, including lowered metabolic rates and short seasonal food availability, are discussed as main evolutionary driving forces shaping Antarctic diversity. In particular, planktotrophic larval stages should be vulnerable to the mismatch of prolonged development and short periods of food avail- ability, selecting against complex life cycles. We hypothesize that larval lecithotrophy and cold tolerance, as recently observed in Subantarctic lithodids, represent, together with other adaptations in the adults, key features among the life-history adaptations of lithodids, potentially enabling them to conquer polar ecosystems. The return of benthic top predators to high Antarctic waters under conditions of climate change would considerably alter the benthic communities. -

Changes in Biomass and Chemical Composition During Lecithotrophic Larval Development of the Southern Stone Crab Paralomis Granulosa
MARINE ECOLOGY PROGRESS SERIES Vol. 257: 189–196, 2003 Published August 7 Mar Ecol Prog Ser Changes in biomass and chemical composition during lecithotrophic larval development of the southern stone crab Paralomis granulosa Javier A. Calcagno1,*, Sven Thatje2, Klaus Anger3, Gustavo A. Lovrich4, Antje Kaffenberger3 1Universidad de Buenos Aires, Facultad de Ciencias Exactas y Naturales, Intendente Güiraldes 2160, Lab 64, 4to Piso, Pab II, Cdad Universitaria C1428EHA, Buenos Aires, Argentina 2Alfred Wegener Institute for Polar and Marine Research, PO Box 120 161, 27515 Bremerhaven, Germany 3Biologische Anstalt Helgoland, Stiftung Alfred Wegener Institute for Polar and Marine Research, 27498 Helgoland, Germany 4Consejo Nacional de Investigaciones Científicas y Técnicas, Centro Austral de Investigaciones Científicas, CADIC, CC 92, V9410BFD Ushuaia, Tierra del Fuego, Argentina ABSTRACT: Changes in biomass and elemental composition (dry mass, W; carbon, C; nitrogen, N; hy- drogen, H) were studied in the laboratory during complete larval and early juvenile development of the southern stone crab Paralomis granulosa (Jacquinot). At 6 ± 0.5°C; total larval development from hatching to metamorphosis lasted ca. 56 d, comprising 2 demersal zoeal stages and a benthic mega- lopa, with mean stage durations of 5, 11 and 45 d, respectively. All larval stages of P. granulosa are lecithotrophic, and first feeding and growth were consistently observed immediately after meta- morphosis to the first juvenile crab stage. Regardless of presence or absence of food, W, C, N, and H decreased throughout larval development. Also the C:N mass ratio decreased significantly, from 7.2 at hatching to 4.2 at metamorphosis, indicating that a large initial lipid store remaining from the egg yolk was gradually utilised as an internal energy source. -

Approach Leaves Earth with Nowhere Left to Fish: UBC Researchers
EMBARGOED UNTIL 5PM EASTERN TIME THU 2 DEC 2010 MEDIA RELEASE | DECEMBER 2, 2010 THE UNIVERSITY OF BRITISH COLUMBIA “No fish left behind” approach leaves Earth UBC Public Affairs with nowhere left to fish: UBC researchers 310 – 6251 Cecil Green Park Rd. Vancouver B.C. Canada V6T 1Z1 The Earth has run out of room to expand fisheries, according to a new study led by University of British Columbia researchers that charts the www.publicaffairs.ubc.ca systemic expansion of industrialized fisheries. Tel: 604.822.3131 Fax: 604.822.2684 In collaboration with the National Geographic Society and published today E-mail: [email protected] in the online journal PLoS ONE, the study is the first to measure the spatial Twitter: @ubcnews expansion of global fisheries. It reveals that fisheries expanded at a rate of one million sq. kilometres per year from the 1950s to the end of the 1970s. The rate of expansion more than tripled in the 1980s and early 1990s – to roughly the size of Brazil’s Amazon rain forest every year. NB: View the study at http://dx.plos.org/10.1371/journal.pone.0015143. Between 1950 and 2005, the spatial expansion of fisheries started from the coastal waters off the North Atlantic and Northwest Pacific, reached into Contacts: the high seas and southward into the Southern Hemisphere at a rate of almost one degree latitude per year. It was accompanied by a nearly five- Brian Lin fold increase in catch, from 19 million tonnes in 1950, to a peak of 90 UBC Public Affairs million tonnes in the late 1980s, and dropping to 87 million tonnes in 2005, Tel: 604.822.2234 Cell: 604.818.5685 according to the study. -

(Isopoda: Bopyridae) on Juveniles of Lithodes Santolla (Magellan Region, Chile): a Spatial Mesoscale Analysis
Lat. Am. J. Aquat. Res., 45(1): 79-93,Infestation 2017 of Pseudione tuberculata on juveniles of Lithodes santolla 79 DOI: 10.3856/vol45-issue1-fulltext-8 Research Article Infestation of Pseudione tuberculata (Isopoda: Bopyridae) on juveniles of Lithodes santolla (Magellan region, Chile): a spatial mesoscale analysis Juan I. Cañete1, Javier A. Díaz-Ochoa1, Tania Figueroa1 & Alvaro Medina1 1Departamento Ciencias y Recursos Naturales, Facultad de Ciencias Universidad de Magallanes, Punta Arenas, Chile Corresponding author: Javier Díaz ([email protected]) ABSTRACT. We document latitudinal patterns of infestation of the bopyrid parasite isopod Pseudione tuberculata on southern king crab Lithodes santolla juveniles (20-77 mm carapace length) recruited to fishing grounds in the southern Chilean fjord system. Seven hundred and fifty individuals were collected by semi- autonomous diving in 11 of 21 sampling locations in the study area, along the western margin of the Magellan region between August and October 2013.The prevalence of P. tuberculata varied between 0 and ~22%, and displayed a spatial pattern associated with three areas: i) northern Beagle Channel (10 to ~22%; lengths between 37 and 47 mm), ii) northwestern Navarino Island without infestations (0%; 26-55 mm), and iii) Piazzi Island- Capitán Aracena Island (0-12%; 50-77 mm). Infestations were independent of host sex, while parasite prevalence decreased with host length. No parasites were observed on crabs longer than 60 mm. A comparison of slopes between linearized length-weight regressions suggests that parasitized individuals had lower weight growth than uninfested individuals. Both southern king crab juvenile density and P. tuberculata prevalence were higher in fishing areas towards Beagle Channel where previous research reported lower average surface water temperatures (<6.5°C) and higher surface water salinity (>30). -

Antarctic Reptant Decapods: More Than a Myth?
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Electronic Publication Information Center Polar Biol (2004) 27: 195–201 DOI 10.1007/s00300-003-0583-z REVIEW Sven Thatje Æ Wolf E. Arntz Antarctic reptant decapods: more than a myth? Received: 21 August 2003 / Accepted: 28 November 2003 / Published online: 10 February 2004 Ó Springer-Verlag 2004 Abstract The impoverished Antarctic decapod fauna is dition of the German Polar Commission to South one of the most conspicuous biodiversity phenomena in Georgia in 1882–1883 (Pfeffer 1887). polar science. Although physiological and ecological Since then, a few new species and records of decapods approaches have tried to explain the reason for the low have been reported from the Southern Ocean (Yaldwyn decapod biodiversity pattern in the Southern Ocean, the 1965; Kirkwood 1984; Tiefenbacher 1990; Thatje 2003). complexity of this problem is still not completely However, known Antarctic decapod diversity remains understood. The scant records of crabs south of poor, represented by approximately only a dozen ben- the Polar Front were always considered as exceptional, thic natant (caridean shrimp) species. Some of those and have mostly been ignored by marine biologists species are known to occur in high abundances on the world-wide, creating one of the most dogmatic para- high-Antarctic Weddell Sea shelf (Arntz and Gorny digms in polar science. We herein review the record of 1991; Arntz et al. 1992; Gorny 1999). both adults and larvae of reptants from the Southern Low temperature is the main physiological impact Ocean. At present, several species of only lithodid crabs on life in polar areas, and results in low metabolic rates maintain considerable adult populations in circum- in polar ectotherms (Clarke 1983; Peck 2001). -

Global Biodiversity Festival the Book 2020
Global Biodiversity Festival — The Book Global Biodiversity Festival The Book 2020 GLOBAL BIODIVERSITY FESTIVAL Fortunately, nature“ is amazingly resilient : places we have destroyed, given time and help, can once again support life, and endangered species can be given a second chance. And there is a growing number of people, especially young people who are aware of these problems and are fighting for the survival of our only home, Planet Earth. We must all join that fight before it is too late. Jane Goodall ”PhD, DBE Founder — The Jane Goodall Institute UN Messenger of Peace GLOBAL BIODIVERSITY FESTIVAL GLOBAL BIODIVERSITY FESTIVAL Foreword The International Day for Biological Diversity gives us About one billion people live in extreme poverty in rural the chance to celebrate the incredible variety of life on areas. Their household income is based on ecosystems Earth, to appreciate nature’s innumerable contributions to and natural goods that make up between 50% and 90% of our everyday lives and to reflect on how it connects us all. the so-called GDP of the poor. Governments should use the occasion of comprehensive recovery plans to build Elizabeth This year’s theme ‘Our solutions are in nature’ economies founded on the conservation and sustainable Maruma Mrema highlights that biodiversity remains the answer to use of nature in the equitable sharing of its benefits. This Executive Secretary, sustainable development challenges. From nature-based will help all, including the most vulnerable. Secretariat of solutions to climate change, food, water security and the Convention on sustainable livelihood, biodiversity remains the basis for We need the world to continue to work towards Biological Diversity a sustainable future. -

About Seafood Watch®
Southern king crab Lithodes santolla ©Monterey Bay Aquarium Argentine waters Traps January 2, 2013 Kelsey James, Consulting Researcher Disclaimer Seafood Watch® strives to ensure all our Seafood Reports and the recommendations contained therein are accurate and reflect the most up-to-date evidence available at time of publication. All our reports are peer- reviewed for accuracy and completeness by external scientists with expertise in ecology, fisheries science or aquaculture. Scientific review, however, does not constitute an endorsement of the Seafood Watch program or its recommendations on the part of the reviewing scientists. Seafood Watch is solely responsible for the conclusions reached in this report. We always welcome additional or updated data that can be used for the next revision. Seafood Watch and Seafood Reports are made possible through a grant from the David and Lucile Packard Foundation. 2 Final Seafood Recommendation Southern king crab (Lithodes santolla) from trap fisheries within Argentine waters is assessed as a Good Alternative. Stock Fishery Impacts Impacts on Manage- Habitat Overall on the other Species ment and Recommendation Stock Ecosystem Rank (Lowest scoring Rank Rank (Score) (Score) species (Score) (Score) Rank*, Subscore, Score) Southern king Trap No other main GOOD crab Yellow Red Yellow species caught ALTERNATIVE (2.64) (2) (3.12) Green, (5,4.5) (2.93) Scoring note – scores range from zero to five where zero indicates very poor performance and five indicates the fishing operations have no significant impact. -

Council of Governments Meeting August 3, 2021
Council of Governments Meeting August 3, 2021 SUBJECT PRESENTATION ON BIVALVE INITIATIVE: “ALL CLAMS ON DECK - RESTORING ESTUARIES AND GROWING COASTAL ECONOMIES” Category AGENDA ITEMS Briefings None Contact and/or Presenter Information Mayor John Chappie, City of Bradenton Beach Ed Chiles, Chiles Group Presenter: Jeff Sedacca Action Requested None Enabling/Regulating Authority Background Discussion A presentation will be made on the Gulfcoast Restoration Initiative, All Clams on Deck - Restoring Estuaries and Growing Coastal Economies. Attorney Review Not Reviewed (No apparent legal issues) Instructions to Board Records None Cost and Funds Source Account Number and Name N/A Amount and Frequency of Recurring Costs N/A GULF COAST RESTORATION INITIATIVE Ed Chiles Affiliates: Gulf Shellfish Institute, Sea & Shoreline Aquatic Restoration, Solutions to Avoid Red Tide (START), Sunnyvale Seafood Company (SSC) ALL CLAMS ON DECK: GETTING ON BOARD TO RESTORE FLORIDA'S ESTUARIES • Paradise under pressure: • Development, Pollution, Runoff • Storms & Hurricanes • Harmful Algal Blooms • Economic consequences • Coastal communities • Commercial and Recreational Fisheries • Florida tourism Photo credit: Capt. Scott Moore BIOLOGICAL MITIGATION STRATEGIES: USING NATURE’S TOOLBOXAquacultured Seagrass & Hard Clams FEDERAL FUNDING & FLORIDA LEGISLATION • $15 M will support proof of concept to research and promote large-scale restoration efforts in 3 National Estuaries • Seagrass restoration & Hard clam deployment • Tampa Bay, Sarasota Bay, Charlotte Harbor • Florida Governor and Legislative Ask • Certify bivalves for mitigation credits and create legislation that provides avenue to proceed • Increase mitigation tools available • Increase capacity of Florida’s shellfish aquaculture industry IT’S TIME TO ACT. Website coming soon: AllClamsOnDeck.org Diatom Initiative Seagrass and Bivalve Restoration Florida has the largest coastal environment in the continental United States. -

Redalyc.Lithodidae Registrados Frente a San Antonio, Chile Central
Investigaciones Marinas ISSN: 0716-1069 [email protected] Pontificia Universidad Católica de Valparaíso Chile Brito, José L. Lithodidae registrados frente a San Antonio, Chile central (Crustacea, Anomura) Investigaciones Marinas, vol. 30, núm. 1, 2002, pp. 57-62 Pontificia Universidad Católica de Valparaíso Valparaíso, Chile Disponible en: http://www.redalyc.org/articulo.oa?id=45630104 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Invest. Mar., Valparaíso, 30(1):Lithodidae 57-62, registrados 2002 frente a San Antonio, Chile central (Crustacea, Anomura) 57 Nota Científica Lithodidae registrados frente a San Antonio, Chile central (Crustacea, Anomura) José Luis Brito M. Museo Municipal de Ciencias Naturales y Arqueología de San Antonio Sanfuentes 2365, Barrancas, San Antonio, Chile E-mail: [email protected] Recibido: 21 septiembre 2001; versión corregida: 14 enero 2002; aceptado: 2 abril 2002 RESUMEN. Se entregan nuevos antecedentes sobre cinco especies de crustáceos lithodidos registrados en el talud continental de Chile central, que son Lithodes panamensis Faxon, 1893, Neolithodes diomedae (Benedict, 1894), Paralomis longipes Faxon, 1893, P. otsuae Wilson, 1990 y Glyptolithodes cristatipes (Faxon, 1893). Palabras claves: crustáceos lithodidos, San Antonio, Chile central. Lithodidae off San Antonio, central Chile (Crustacea, Anomura) ABSTRACT. New data about five species of crustaceans Lithodidae, recorded from Central Chile continental slope are given, Lithodes panamensis Faxon, 1893, Neolithodes diomedeae (Benedict, 1894), Paralomis longipes Faxon, 1893, P. otsuae Wilson, 1990 and Glyptolithodes cristatipes (Faxon, 1893). -
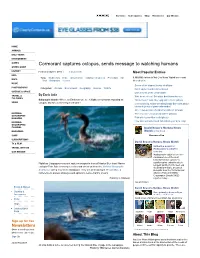
Cormorant Captures Octopus, Sends Message to Watching Humans
Site Index Subscriptions Shop Newsletters Our Mission HOME ANIMALS DAILY NEWS ENVIRONMENT GAMES Cormorant captures octopus, sends message to watching humans GREEN GUIDE HISTORY Posted on April 8, 2010 | 0 Comments Most Popular Entries KIDS Tags: biodiversity birds conservation endangered species Enric Sala fish 1,500,000 visitors to Nat Geo News Watch were most MAPS food Galapagos oceans interested in: MUSIC Seven of the biggest beasts of all time PHOTOGRAPHY Categories: Animals Environment Geography Science Wildlife Giant spider found in Israel desert SCIENCE & SPACE Dark secrets of the Devil's Bible By Enric Sala TRAVEL & Why do we sleep? Scientists don't know for sure CULTURES Galapagos Islands--Where on Earth can one see a flightless cormorant capturing an "Watermelon" tapir, like a pig with a trunk (photo) VIDEO octopus, and two orcas killing a sea turtle? Croc-grabbing, snake-wrestling Brady Barr talks about his work (photo of giant salamander) One in two primates headed for oblivion (photos) NATIONAL The very last "uncontacted tribes" (photos) GEOGRAPHIC MAGAZINE Fish with human-like teeth (photo) How asteroid obliterated 160-million-year dino reign NATIONAL GEOGRAPHIC CHANNEL David Braun's NatGeo News MAGAZINES Watch on Facebook SHOP Become a Fan SUBSCRIPTIONS David Braun's NatGeo News Watch TV & FILM Extinction nears for TRAVEL WITH US Madagascar's radiated OUR MISSION tortoise Madagascar's radiated tortoise-- considered one of the most beautiful tortoise species--is Flightless Galapagos cormorant captures octopus in front of Mission Blue team! Marine rapidly nearing extinction due to rampant hunting for its meat and ecologist Enric Sala is among scientists and others on board the National Geographic the illegal pet trade, a team of Endeavour, sailing around the Galapagos. -

Particle Dynamics in Ushuaia Bay (Tierra Del Fuego)-Potential Effect on Dissolved Oxygen Depletion
water Article Particle Dynamics in Ushuaia Bay (Tierra del Fuego)-Potential Effect on Dissolved Oxygen Depletion Ximena Flores Melo 1,*, Jacobo Martín 1, Lounes Kerdel 2, François Bourrin 2 , Cristina Beatriz Colloca 3, Christophe Menniti 2 and Xavier Durrieu de Madron 2,* 1 Laboratorio de Oceanografía, Centro Austral de Investigaciones Científicas (CADIC-CONICET), Bernardo Houssay 200, V9410CAB Ushuaia, Argentina; [email protected] 2 CEFREM, CNRS, Université de Perpignan Via Domitia, 52 avenue Paul Alduy, 66860 Perpignan, France; [email protected] (L.K.); [email protected] (F.B.); [email protected] (C.M.) 3 Institute of Polar Sciences, Natural Resources and Environment, Universidad Nacional de Tierra del Fuego, Fuegia Basket 251, V9410BXE Ushuaia, Argentina; [email protected] * Correspondence: ximenafl[email protected] (X.F.M.); [email protected] (X.D.d.M.) Received: 21 December 2019; Accepted: 19 January 2020; Published: 22 January 2020 Abstract: This study examines the distribution and seasonal evolution of hydrographic, hydrodynamic, and nepheloid layers in Ushuaia Bay and the submerged glacial valley that connects it to the Beagle Channel. The hydrographic structure is highly seasonal, with a total mixing of the water column in winter and the appearance of a pycnocline between 50 and 70 m deep from spring to late autumn, mainly due to desalination. A counter-clockwise current sweeps the entire bay regardless of the season or phase of the tide. This current is at its maximum in the surface layer, allowing the rapid renewal of the bay’s waters, while deep currents are weak and imply a slow renewal of the valley’s waters.