Predicting the Areas of Suitable Distribution for Zelkova Serrata in China Under Climate Change
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

Landscape Review Committee Community Development Center, 231 NE 5Th Street October 24, 2018 12:00 PM
City of McMinnville Planning Department 231 NE Fifth Street McMinnville, OR 97128 (503) 434-7311 www.mcminnvilleoregon.gov Landscape Review Committee Community Development Center, 231 NE 5th Street October 24, 2018 12:00 PM Committee Members Agenda Items Rob Stephenson 1. Call to Order Chair 2. Citizen Comments 3. Approval of Minutes Sharon Gunter 4. Action Items Vice-Chair 5. Discussion Items A. McMinnville street tree list update Josh Kearns 6. Old/New Business 7. Committee Member Comments RoseMarie Caughran 8. Staff Comments 9. Adjournment Tim McDaniel The meeting site is accessible to handicapped individuals. Assistance with communications (visual, hearing) must be requested 24 hours in advance by contacting the City Manager (503) 434-7405 – 1-800-735-1232 for voice, or TDY 1-800-735-2900. *Please note that these documents are also on the City’s website, www.mcminnvilleoregon.gov. You may also request a copy from the Planning Department. City of McMinnville Planning Department 231 NE Fifth Street McMinnville, OR 97128 (503) 434-7311 www.mcminnvilleoregon.gov STAFF REPORT DATE: October 24, 2018 TO: Landscape Review Committee Members FROM: Jamie Fleckenstein, PLA, Associate Planner SUBJECT: Agenda Item 5A: McMinnville street tree list update Report in Brief: The City of McMinnville requires species of street trees planted within public rights-of-way to be chosen from an approved street tree list. The purpose of this discussion is to determine if an update and revision to the approved street tree list is needed. Background: The current McMinnville Street Tree List was adopted by resolution on May 10, 2016. The list provides general characteristics desirable in street trees in McMinnville and lists several recommended street trees generally acceptable for use as street trees. -

Zelkova Serrata 'Green Vase'
Fact Sheet ST-678 October 1994 Zelkova serrata ‘Green Vase’ ‘Green Vase’ Japanese Zelkova1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION ‘Green Vase’ somewhat resembles the vase shape of American Elms, is more upright in habit and tolerant of pollution, makes a great city street tree and produces a taller and narrower tree than ‘Village Green’ Zelkova (Fig. 1). Zelkova is often listed as a replacement for American Elm since it has roughly the same vase shape and grows 70 to 80 feet tall with a 50 to 60-foot spread. But no tree will truly match the grace and elegance of the American Elm. Zelkova is massive, with the trunk capable of growing to four feet or more in diameter. It has a moderate growth rate and likes a sunny exposure. Branches are more numerous and smaller in diameter than American Elm. Major branches grow very upright and provide easy clearance for tall vehicles below making it quite suitable as a street tree. Leaves are 1.5 to 4 inches long, turning a brilliant burnt umber in the fall. GENERAL INFORMATION Scientific name: Zelkova serrata ‘Green Vase’ Figure 1. Young ‘Green Vase’ Japanese Zelkova. Pronunciation: zell-KOE-vuh sair-AY-tuh Common name(s): ‘Green Vase’ Japanese Zelkova, sidewalk cutout (tree pit); residential street tree; tree ‘Green Vase’ Saw-Leaf Zelkova has been successfully grown in urban areas where air Family: Ulmaceae pollution, poor drainage, compacted soil, and/or USDA hardiness zones: 5B through 8 (Fig. 2) drought are common Origin: not native to North America Availability: generally available in many areas within Uses: large parking lot islands (> 200 square feet in its hardiness range size); wide tree lawns (>6 feet wide); medium-sized parking lot islands (100-200 square feet in size); medium-sized tree lawns (4-6 feet wide); recommended for buffer strips around parking lots or for median strip plantings in the highway; shade tree; 1. -

Invasive Plant List
NON-NATIVE INVASIVE PLANTS OF ARLINGTON COUNTY, VIRGINIA While up to 40% of the plants found in a typical urban environment are non-native species, a relatively small number of these “alien” plants are known to represent an ecological threat to the natural environment (parks, woodlands, and backyards). Known as “invasive species”, these non-natives will spread from urban plantings into natural areas, eliminate native species, alter natural plant communities, and degrade the environment. The following plants have been documented as invasive species in Arlington. Known invasive plant species should not be planted as part of any Arlington County sponsored project. This list will be periodically reviewed by the Invasive Plant Coordinator (DPR) and updated by Version (date). Invasive Plant Species List Acer spp.: campestre, tataricum var. ginnala Hedge, Amur maple Threat Acer spp.: palmatum, plantanoides, pseudoplatanus Japanese, Norway, Sycamore maple Invasive Actinidia arguta Hardy kiwi Threat Aegopodium podagraria Goutweed Invasive Agrostis capillaris Colonial bent-grass Invasive Ailanthus altissima Tree of Heaven Invasive Akebia quinata Five-leaved akebia Invasive Albizia julibrissin Mimosa Invasive Aldrovanda vesiculosa* Waterwheel Threat Alliaria petiolata Garlic mustard Invasive Alternanthera philoxeroides Alligator weed Invasive Ampelopsis brevipedunculata Porcelainberry Invasive Aralia elata Japanese angelica tree Invasive Artemisia vulgaris Mugwort Invasive Arthraxon hispidus var. hispidus Hairy jointgrass Invasive Arum italicum -
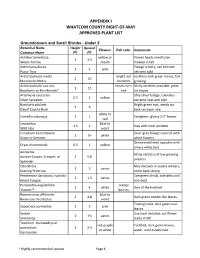
Whatcom County's Approved Plant List
APPENDIX I WHATCOM COUNTY RIGHT-OF-WAY APPROVED PLANT LIST Groundcovers and Small Shrubs - Under 2' Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Achillea tomentosa yellow or Flower heads need to be 1 3-5 Wooly Yarrow cream mowed in fall Antennaria dioica Foliage is furry, can tolerate 1 2 pink Pussy Toes extreme cold Arctostaphylos media bright red Leathery dark green leaves, fast 2 10 Manzanita Media branches growing Arctostaphylos uva ursi leaves turn Many varieties available, great 1 15 Bearberry or Kinnikinnick* red for slopes Artemesia caucasica Silky silver foliage, tolerates 0.5 2 yellow Silver Spreader extreme heat and cold Baccharis pilularis Bright green mat, needs cut 2 6 Dwarf Coyote Bush back once per year white to Camellia sasanqua 1 1 Evergreen, glossy 2-3" leaves red Ceanothus blue to 1.5 2 Stay with local varieties Wild Lilac violet Cerastium tomentosum Silver gray foliage covered with 1 5+ white Snow-in-Summer white flowers Ornamental seed capsules with Dryas drummondii 0.5 1 yellow silvery white tails Juniperus Many varieties of low growing Juniper-Carpet, Creeper, or 2 5-8 junipers Spreader Oenothera May die back in severe winters, 1 2 varies Evening Primrose come back strong Penstemon davidsonii, rupicola Evergreen shrub, tolerates cold 2 1.5 varies Beard Tongue and heat Pyracantha augustifolia orange 1 4 white One of the hardiest 'Gnome'* berries Rosmariunus officinalis blue to 2 4-8 Dark green needle-like leaves Rosemary 'Prostratus' violet Trailing habit, dark green oval Saponaria ocymoides 1 3 pink leaves Sedum Use local varieties, cut flower 2 1-5 varies Stonecrop stalks in fall Teucrium chamaedrys or red-purple Toothed, dark green leaves, postratium 1 2-3 or white water until established Germander * Highly-recommended species Page 1 Thymus white to 2 1-2 Many varieties Thyme purple Shrubs Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Arctostaphylos bright red Shiny dark green leaves turn 2-15 20 varies Manzanita berries maroon in fall. -
Elmcare.Com - All About Elm Trees and Elm Tree Care
Elmcare.com - All about elm trees and elm tree care. Home | Elm Care Products | Register your Elm | Forum Last Update 17/12/01 Customized Elm Tree Care Kits Did you know a new wild bird seed has been developed and tested that Custom care kits include actually repels squirrels? How Trees Work specialized and innovative soil Click here to learn about Squirrel Proof Wild Bird treatments designed to promote About Elm Trees Seed. root development and the long- Caring for Your Elm term health and vitality of your Elm Tree Diseases elm. A healthier elm will be better able to fight against Dutch elm disease. Elm Tree Links more Quick Elm Facts Elm Tree Registry Visit TreeHelp.com for all of your tree and shrub Register your elm tree to receive customized care care needs advice...more. Return of the Stately Elm Writer and broadcaster Jamie Swift examines the Canadian city of Winnipeg's struggle to combat Dutch elm disease. Through the work of community groups like the Coalition to Save the Elms and innovative technology, the city has achieved substantial success... more. http://www.elmcare.com/index.htm (1 of 2) [2/27/02 10:29:57 PM] Elmcare.com - All about elm trees and elm tree care. New Treatments for Elms in History Elm Tree Links Dutch Elm Disease? Look at elms in the context of Links to a growing community of Researchers examine new ways to human history. academics, homeowners and fight this devastating disease. professional tree care experts. Elms in Literature Elm Species Quick Elm Facts Read what some of the world's Elms come in many sizes and leading poets and authors have Discover something new and shapes...learn about them all. -

Zelkova Serrata.Cdr
Japanese Gray-Bark Elm - Zelkova serrata General information: Zelkova is often listed as a replacement for American Elm since it has roughly the same vase shape and grows 90 to 100 feet tall with a 60 to 80 foot spread. Zelkova is massive, with the trunk capable of growing to four feet or more in diameter. It has a moderate growth rate and likes a sunny exposure. Branches are more numerous and smaller in diameter than American Elm. Leaves are 1.5 to 4 inches long, turning a brilliant yellow, orange, or burnt umber in the fall. This elm is a native of Japan and China and is related to the Ulmus genus, which is the genus of the European and American elms. It is deciduous with small ovate, serrated, pointed leaves and smooth gray bark. It is a vigorous grower and is most often used for broom and group plantings. Root-over- rock plantings are also common. The gray-bark elm is considered by some to be the classic broom style tree. Family: Ulmaceae Lighting: Full sun to part shade. During summer in southern part of USA, do not expose the tree to direct sun during the heat of the day. Temperature: Zones 5 through 8. Make sure the tree has good winter protection. Watering: Allow the soil to dry slightly between waterings. Spray the foliage with water daily during the summer. Feeding: Feed every 20-30 days with a slow-acting fertilizer during spring and again from late summer through mid-autumn. If you prefer to use chemical fertilizers, feed every other week using a half-strength solution of a balanced fertilizer such as Peter's 20-20-20. -

Suitability of Thirteen Different Host Species for Elm Leaf Beetle, Xanthogaleruca Luteola (Coleoptera: Chrysomelidae) 1
Suitability of Thirteen Different Host Species for Elm Leaf Beetle, Xanthogaleruca luteola (Coleoptera: Chrysomelidae) 1 Richard W. Hall, 2 Alden M. Townsend, 3 and Jack H. Barger4 Department of Entomology The Ohio State University 1735 Neil Avenue Columbus, OH 43210 r-------------------Abstract---------------- Thirteen different host species for elm leaf beetle, Xanthogaleruca luteola (Muller), were assayed to determine their relative suitability. Species examined were Ulmus parv1folia Jacq., U. thomasii Sarg., U. laevis Pall., U. wilsoniana Schneid., U. americana L., U. japonica Sarg., U. pumila L., U. rubra Muhl., U. laciniata Mayr., U. glabra Huds., U. carpinifolia Gledisch, Zelkova serrata Makino, and '204,' a hybrid of U. carpinifolia x U. parvifolia. Suitability was determined by feeding adults excised foliage from a tree and measuring mortality and fecundity during a two wk period. Significant differences in beetle mortality and fecundity occurred among hosts. In general, European elms were better hosts than American or Asiatic species. Suitability of a European x Asiatic hybrid fell between that of the parent species. Index words: host plant resistance, insect-plant interactions, Ulm us spp., Zelkova serrata, elm leaf beetle, Xanthogaleruca luteola Introduction Makino, and '204,' a hybrid of V. carpinifolia x V. par The elm leaf beetle (ELB), Xanthogaleruca luteola vifolia were tested for suitability as hosts for ELB. Ten trees (Muller), was introduced into the United States from Europe were examined for each elm except V. rubra and '204' in the 1830's ( 1). Since its introduction, it has become a where a single tree was examined and V. laciniata where major defoliator of elms in urban environments throughout two trees were examined. -

Zelkova Serrata
Zelkova serrata (Thun.) Makino • Zelkova serrata (Thun.) Makino Japanese Zelkova/Keyaki (Japan)/Ju shu (China)/Neutinamu (Korea) IUCN Red List of Threatened Species: Not Evaluated Zelkova serrata exhibits the larg- est geographical range among the members in the genus. Its densely grained and rot-resistant hardwood is widely sought after in construction and shipbuilding, and for the pro- duction of tool handles and superior 8 quality furniture. The recent discov- ery of compounds in twig extracts of this species that are relevant to cancer treatment, may offer new op- portunities for Z. serrata to become a major medicinal plant in the future. 9 10 1-2. Upper surface and underside of a sterile branchlet (hrs) 3-4. Upper surface and underside of leaves (hrs) 5-6. Closer view of the 1 2 upper surface and underside of a leaf (hrs) 7. Underside of a fertile branchlet (hrs) 3 4 8. Fruits (hrs) 9-10. Young branches of Z. serrata are brownish purple and glabrous 11 12 13 (eg, gk) 11. Seedlings of Z. serrata on the forest floor. 5 6 7 Chichibu, Honshu Island, DISTRIBUTION Japan (sb) In Japan, Z. serrata naturally occurs from the south of Kyushu Island 12. Stem of Z. serrata. (Kagoshima) through Shikoku Island to the extreme north of Honshu HABIT Chichibu, Honshu Island, Island (Aomori). For centuries, this species has been widely planted Z. serrata is a large tree that can grow up to 30 m and reach 1 m in diameter, Japan (sb) as an ornamental tree in parks, near homes and along streets and al- and produces an exfoliating grayish white to grayish brown bark upon ageing. -

Biogeographic Overview of Ulmaceae: Diversity, Distribution, Ecological Preferences, and Conservation Status
plants Article Biogeographic Overview of Ulmaceae: Diversity, Distribution, Ecological Preferences, and Conservation Status Yann Fragnière 1 , Yi-Gang Song 2 , Laurence Fazan 1, Steven R. Manchester 3, Giuseppe Garfì 4 and Gregor Kozlowski 1,2,5,* 1 Department of Biology and Botanic Garden, University of Fribourg, Chemin du Musée 10, CH-1700 Fribourg, Switzerland; [email protected] (Y.F.); [email protected] (L.F.) 2 Eastern China Conservation Centre for Wild Endangered Plant Resources, Shanghai Chenshan Botanical Garden, 3888 Chenhua Road, Songjiang, Shanghai 201602, China; [email protected] 3 Florida Museum of Natural History, University of Florida, 1659 Museum Rd, Gainesville, FL 32611, USA; steven@flmnh.ufl.edu 4 Institute of Biosciences and BioResources—National Research Council, Corso Calatafimi 414, 90129 Palermo, Italy; giuseppe.garfi@ibbr.cnr.it 5 Natural History Museum Fribourg, Chemin du Musée 6, CH-1700 Fribourg, Switzerland * Correspondence: [email protected]; Tel.: +41-26-300-88-42 Abstract: The elm family (Ulmaceae) is a woody plant group with important scientific, societal, and economic value. We aim to present the first biogeographic synthesis investigating the global diversity, distribution, ecological preferences, and the conservation status of Ulmaceae. A literature review was performed to explore the available data for all extant species. Our study made it possible to map the actual global distribution of Ulmaceae with high precision, and to elucidate the centers of diversity, located mainly in China and in the southeastern USA. A detailed comparative analysis Citation: Fragnière, Y.; Song, Y.-G.; of the macroclimatic niche for each species was produced, which shows the general biogeographic Fazan, L.; Manchester, S.R.; Garfì, G.; pattern of the family and pinpoints the outlier species. -

Spring 2018 Parkway Tree Planting
VILLAGE OF LISLE . Botanical Name: Carpinus caroliniana . Fall Color: Red-orange . Mature Height: 25 ft. Mature Width: 20 ft. Also known as Ironwood, Blue Beech or Musclewood Photo curtesy of: http://springgrovenursery.com/shade-trees/our- gallery/carpinus-hornbeam/american-hornbeam . Botanical Name: Sringa pekinesis ‘Zhang Zhiming’ . Fall Color: Yellow - organge . Mature Height: 15-20 ft. Mature Width: 10-15 ft. Fragrant yellow flowers in early summer Photo curtesy of: https://www.mortonarb.org/trees-plants/tree-plant-descriptions/beijing-gold%E2%84%A2-peking-lilac . Botanical Name: Syringa pekinesis ‘Morton’ . Fall Color: Yellow . Mature Height: 20-30 ft. Mature Width: 15-25 ft. Amber – colored bark that peels Photo curtesy of: https://www.mortonarb.org/trees-plants/tree-plant-descriptions/china-snow%E2%84%A2-peking-lilac year round . Fragrant white flowers in early summer Photo curtesy of: https://www.mortonarb.org/trees-plants/tree-plant-descriptions/peking-lilac . Botanical Name: Quercus muehlenbergii . Fall Color: Yellow - brown . Mature Height: 45 ft. Mature Width: 45 ft. Rounded – open form Photo curtesy of: https://springgrovenursery.com/product/chinkapin-oak/ . Botanical name: Platanus x acerifolia ‘Morton Circle’ . Fall color: yellow – brown . Mature height: 60 ft. Mature width: 30 ft. Resistant to anthacnose and frost cracking . More uniform and upright growth habit Photo curtesy of: http://plants.oaklandnursery.com/12130001/Plant/16673/Exclamation!_London_Planetree . Botanical Name: Zelkova serrata ‘Halka’ . Fall Color: Yellow . Mature Height: 50 ft. Mature Width: 50 ft. Vase – shaped growth habit Photo curtesy of: https://www.mortonarb.org/trees-plants/tree-plant-descriptions/japanese-zelkova#destination . Botanical Name: Catalpa erubescens ‘Purpurea’ . -
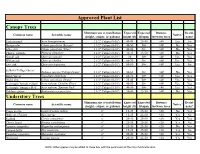
Canopy Trees Understory Trees Approved Plant List
Approved Plant List Canopy Trees Minimum size at installation Expected Expected Distance Decid- Common name Scientific name Native? (height, caliper, or gallons) height (ft) lifespan between trees uous? Trident maple Acer buergerianum 2 1/2" Caliper (8-10') 40-45 25-50 <40 Yes No Bosque elm Ulmus parvifolia 'Bosque' 2 1/2" Caliper (8-10') 40-50 50+ <40' No Yes Allee elm Ulmus parvifolia 'Allee' 2 1/2" Caliper (8-10') 40-50 50+ <40' No Yes Chinese pistache Pistacia chinensis 2 1/2" Caliper (8-10') 30-35 25-50 <40' No Yes Nuttall oak Quercus nattallii 2 1/2" Caliper (8-10') 50 50+ <40' Yes Yes Willow oak Quercus phellos 2 1/2" Caliper (8-10') 60-70 50+ <40' Yes Yes Live oak Quercus virginiana 2 1/2" Caliper (8-10') 60-80 50+ <40' Yes No Zelkova 'Village Green' Zelkova serrata 'Village Green' 2 1/2" Caliper (8-10') 50-60 50+ <40' No Yes Bald cypress Taxodium distichum 2 1/2" Caliper (8-10') 60-70 50+ <40' Yes Yes Drake elm Ulmus parvifolia 'Drake' 2 1/2" Caliper (8-10') 35-45 50+ <40' Yes Yes Red maple 'October Glory' Acer rubrum 'October Glory' 2 1/2" Caliper (8-10') 40-50 50+ <40' Yes Yes Red maple 'Summer Red' Acer rubrum 'Summer Red' 2 1/2" Caliper (8-10') 40-50 50+ <40 Yes Yes Golden raintree Koelreuteria paniculata 2 1/2" Caliper (8-10') 30-40 25-50 <40' No Yes Understory Trees Minimum size at installation Expected Expected Distance Decid- Common name Scientific name Native? (height, caliper, or gallons) height (ft) lifespan between trees uous? Crape myrtle Lagerstroemia indica 6' 15-35 25-50 <20 No Yes Holly sp.