Comparison Laboratory Methods for Detection of Hookworms Infection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca Volvulus Excretory Secretory Products
pathogens Review The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca volvulus Excretory Secretory Products Luc Vanhamme 1,*, Jacob Souopgui 1 , Stephen Ghogomu 2 and Ferdinand Ngale Njume 1,2 1 Department of Molecular Biology, Institute of Biology and Molecular Medicine, IBMM, Université Libre de Bruxelles, Rue des Professeurs Jeener et Brachet 12, 6041 Gosselies, Belgium; [email protected] (J.S.); [email protected] (F.N.N.) 2 Molecular and Cell Biology Laboratory, Biotechnology Unit, University of Buea, Buea P.O Box 63, Cameroon; [email protected] * Correspondence: [email protected] Received: 28 October 2020; Accepted: 18 November 2020; Published: 23 November 2020 Abstract: Nematodes constitute a very successful phylum, especially in terms of parasitism. Inside their mammalian hosts, parasitic nematodes mainly dwell in the digestive tract (geohelminths) or in the vascular system (filariae). One of their main characteristics is their long sojourn inside the body where they are accessible to the immune system. Several strategies are used by parasites in order to counteract the immune attacks. One of them is the expression of molecules interfering with the function of the immune system. Excretory-secretory products (ESPs) pertain to this category. This is, however, not their only biological function, as they seem also involved in other mechanisms such as pathogenicity or parasitic cycle (molting, for example). Wewill mainly focus on filariae ESPs with an emphasis on data available regarding Onchocerca volvulus, but we will also refer to a few relevant/illustrative examples related to other worm categories when necessary (geohelminth nematodes, trematodes or cestodes). -

Hookworm (Ancylostomiasis)
Hookworm (ancylostomiasis) Hookworm (ancylostomiasis) rev Jan 2018 BASIC EPIDEMIOLOGY Infectious Agent Hookworm is a soil transmitted helminth. Human infections are caused by the nematode parasites Necator americanus and Ancylostoma duodenale. Transmission Transmission primarily occurs via direct contact with fecal contaminated soil. Soil becomes contaminated with eggs shed in the feces of an individual infected with hookworm. The eggs must incubate in the soil for several days before they become infectious and are able to be transmitted to another person. Oral transmission can sometimes occur from consuming improperly washed food grown or exposed to fecal contaminated soil. Transmission can also occur (rarely) between a mother and her fetus/infant via infected placental or mammary tissue. Incubation Period Eggs must incubate in the soil for 5-10 days before they mature into infectious filariform larvae that can penetrate the skin. Within the first 10 days following penetration of the skin filariform larvae will migrate to the lungs and occasionally cause respiratory symptoms. Three to five weeks after skin penetration the larvae will migrate to the intestinal tract where they will mature into an adult worm. Adult worms may live in the intestine for 1-5 years depending on the species. Communicability Human to human transmission of hookworm does NOT occur because part of the worm’s life cycle must be completed in soil before becoming infectious. However, vertical transmission of dormant filariform larvae can occur between a mother and neonate via contaminated breast milk. These dormant filariform larvae can remain within in a host for months to years. Soil contamination is perpetuated by fecal contamination from infected individuals who can shed eggs in feces for several years after infection. -

Performance of Two Serodiagnostic Tests for Loiasis in A
Performance of two serodiagnostic tests for loiasis in a Non-Endemic area Federico Gobbi, Dora Buonfrate, Michel Boussinesq, Cédric Chesnais, Sébastien Pion, Ronaldo Silva, Lucia Moro, Paola Rodari, Francesca Tamarozzi, Marco Biamonte, et al. To cite this version: Federico Gobbi, Dora Buonfrate, Michel Boussinesq, Cédric Chesnais, Sébastien Pion, et al.. Perfor- mance of two serodiagnostic tests for loiasis in a Non-Endemic area. PLoS Neglected Tropical Dis- eases, Public Library of Science, 2020, 14 (5), pp.e0008187. 10.1371/journal.pntd.0008187. inserm- 02911633 HAL Id: inserm-02911633 https://www.hal.inserm.fr/inserm-02911633 Submitted on 4 Aug 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. PLOS NEGLECTED TROPICAL DISEASES RESEARCH ARTICLE Performance of two serodiagnostic tests for loiasis in a Non-Endemic area 1 1 2 2 Federico GobbiID *, Dora Buonfrate , Michel Boussinesq , Cedric B. Chesnais , 2 1 1 1 3 Sebastien D. Pion , Ronaldo Silva , Lucia Moro , Paola RodariID , Francesca Tamarozzi , Marco Biamonte4, Zeno Bisoffi1,5 1 IRCCS Sacro -

61% of All Human Pathogens Are Zoonotic (Passed from Animals to Humans), and Many Are Transmitted Through Inhaling Dust Particles Or Contact with Animal Wastes
Zoonotic Diseases Fast Facts: 61% of all human pathogens are zoonotic (passed from animals to humans), and many are transmitted through inhaling dust particles or contact with animal wastes. Some of the diseases we can get from our pets may be fatal if they go undetected or undiagnosed. All are serious threats to human health, but can usually be avoided by observing a few precautions, the most effective of which is washing your hands after touching animals or their wastes. Regular visits to the veterinarian for prevention, diagnosis, and treatment of zoonotic diseases will help limit disease in your pet. Source: http://www.cdc.gov/healthypets/ Some common zoonotic diseases humans can get through their pets: Zoonotic Disease & its Effect on How Contact is Made Humans Bartonellosis (cat scratch disease) – an Bartonella bacteria are transferred to humans through infection from the bacteria Bartonella a bite or scratch. Do not play with stray cats, and henselae that causes fever and swollen keep your cat free of fleas. Always wash hands after lymph nodes. handling your cat. Capnocytophaga infection – an Capnocytophaga canimorsus is the main human infection caused by bacteria that can pathogen associated with being licked or bitten by an develop into septicemia, meningitis, infected dog and may present a problem for those and endocarditis. who are immunosuppressed. Cellulitis – a disease occurring when Bacterial organisms from the Pasteurella species live bacteria such as Pasteurella multocida in the mouths of most cats, as well as a significant cause a potentially serious infection of number of dogs and other animals. These bacteria the skin. -

Parasitic Infections: Minnesota Refugee Health Provider Guide
Parasitic Infections at a Glance Minnesota Initial Refugee Health Assessment Yes No If yes, was Eosinophilia present? Yes No Results pending If yes, was further evaluation done? Yes No Yes No If yes, was Eosinophilia present? Yes No Results pending If yes, was further evaluation done? Yes No ( one) Yes No If why not? _________________________________________ Done Results Pending Not done Negative Positive; treated: ___yes ___no Indeterminate Results Pending Not done Negative Positive; treated: ___yes ___no Indeterminate Results Pending Not done ( one) Yes No If why not? _________________________________________ No parasites found Results Pending Nonpathogenic parasites found Blastocystis; treated: ___yes ___no Not done Pathogenic parasite(s) found () Done Results Pending Not done Treated? Yes No Treated? Yes No Treated? Yes No Species: __________________________ Treated? Yes No Treated? Yes No Treated? Yes No Treated? Yes No Negative Positive; treated: ___yes ___no Indeterminate Results Pending Not done Treated? Yes No Treated? Yes No (specify)Treated? Yes No Treated? Yes No Treated? Yes No _______________________ If not treated, why not? Negative Positive; treated: ___yes ___no Indeterminate Results Pending Not done (check one) Not screened for malaria (e.g., No symptoms and history not suspicious of malaria) Screened, no malaria species found in blood smears Screened, malaria species found (please -
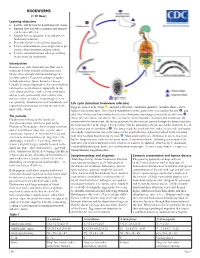
HOOKWORMS (1 CE Hour) Learning Objectives !! List the Risk Factors for Hookworm Infections
HOOKWORMS (1 CE Hour) Learning objectives ! List the risk factors for hookworm infections. ! Explain how and where humans and animals can become infected. ! Explain how to diagnose, treat and prevent hookworm infection. ! Describe the life cycle of these parasites. ! List recommendations you can provide to pet owners about treatment and prevention. ! List the contraindications when prescribing medications for hookworm. Introduction Zoonoses are infectious diseases that can be transmitted from animals to humans, most likely when animals and human beings are in close contact. Causative groups of agents include parasites, fungi, bacteria, viruses. Usually of minor importance, they nevertheless can lead to severe disease, especially in the very young and those with certain conditions, and to death, particularly with certain viral diseases (such as rabies, hemorrhagic fevers, encephalitis). Hookworms exist worldwide and Life cycle (Intestinal hookworm infection) cross from carnivorous animals to man in all Eggs are passed in the stool , and under favorable conditions (moisture, warmth, shade), larvae parts of the world. hatch in one to two days. The released rhabditiform larvae grow in the feces and/or the soil , and The parasite after 5 to 10 days (and two molts) they become filariform (third-stage) larvae that are infective . These infective larvae can survive three to four weeks in favorable environmental conditions. On Hookworms belong to the family of contact with the human host, the larvae penetrate the skin and are carried through the blood vessels to Ancylostomatidae which are part of the the heart and then to the lungs. They penetrate into the pulmonary alveoli, ascend the bronchial tree to Phylum of Nematodes: Ancylostoma caninum the pharynx and are swallowed . -

Developing Vaccines to Combat Hookworm Infection and Intestinal Schistosomiasis
REVIEWS Developing vaccines to combat hookworm infection and intestinal schistosomiasis Peter J. Hotez*, Jeffrey M. Bethony*‡, David J. Diemert*‡, Mark Pearson§ and Alex Loukas§ Abstract | Hookworm infection and schistosomiasis rank among the most important health problems in developing countries. Both cause anaemia and malnutrition, and schistosomiasis also results in substantial intestinal, liver and genitourinary pathology. In sub-Saharan Africa and Brazil, co-infections with the hookworm, Necator americanus, and the intestinal schistosome, Schistosoma mansoni, are common. The development of vaccines for these infections could substantially reduce the global disability associated with these helminthiases. New genomic, proteomic, immunological and X-ray crystallographic data have led to the discovery of several promising candidate vaccine antigens. Here, we describe recent progress in this field and the rationale for vaccine development. In terms of their global health impact on children and that combat hookworm and schistosomiasis, with an pregnant women, as well as on adults engaged in subsist- emphasis on disease caused by Necator americanus, the ence farming, human hookworm infection (known as major hookworm of humans, and Schistosoma mansoni, ‘hookworm’) and schistosomiasis are two of the most the primary cause of intestinal schistosomiasis. common and important human infections1,2. Together, their disease burdens exceed those of all other neglected Global distribution and pathobiology tropical diseases3–6. They also trap the world’s poorest Hookworms are roundworm parasites that belong to people in poverty because of their deleterious effects the phylum Nematoda. They share phylogenetic simi- on child development and economic productivity7–9. larities with the free-living nematode Caenorhabditis Until recently, the importance of these conditions as elegans and with the parasitic nematodes Nippostrongylus global health and economic problems had been under- brasiliensis and Heligmosomoides polygyrus, which are appreciated. -

Neglected Tropical Diseases: Epidemiology and Global Burden
Tropical Medicine and Infectious Disease Review Neglected Tropical Diseases: Epidemiology and Global Burden Amal K. Mitra * and Anthony R. Mawson Department of Epidemiology and Biostatistics, School of Public Health, Jackson State University, Jackson, PO Box 17038, MS 39213, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-601-979-8788 Received: 21 June 2017; Accepted: 2 August 2017; Published: 5 August 2017 Abstract: More than a billion people—one-sixth of the world’s population, mostly in developing countries—are infected with one or more of the neglected tropical diseases (NTDs). Several national and international programs (e.g., the World Health Organization’s Global NTD Programs, the Centers for Disease Control and Prevention’s Global NTD Program, the United States Global Health Initiative, the United States Agency for International Development’s NTD Program, and others) are focusing on NTDs, and fighting to control or eliminate them. This review identifies the risk factors of major NTDs, and describes the global burden of the diseases in terms of disability-adjusted life years (DALYs). Keywords: epidemiology; risk factors; global burden; DALYs; NTDs 1. Introduction Neglected tropical diseases (NTDs) are a group of bacterial, parasitic, viral, and fungal infections that are prevalent in many of the tropical and sub-tropical developing countries where poverty is rampant. According to a World Bank study, 51% of the population of sub-Saharan Africa, a major focus for NTDs, lives on less than US$1.25 per day, and 73% of the population lives on less than US$2 per day [1]. In the 2010 Global Burden of Disease Study, NTDs accounted for 26.06 million disability-adjusted life years (DALYs) (95% confidence interval: 20.30, 35.12) [2]. -

Controlling Disease Due to Helminth Infections
During the past decade there have been major efforts to plan, Controlling disease due to helminth infections implement, and sustain measures for reducing the burden of human ControllingControlling diseasedisease disease that accompanies helminth infections. Further impetus was provided at the Fifty-fourth World Health Assembly, when WHO duedue toto Member States were urged to ensure access to essential anthelminthic drugs in health services located where the parasites – schistosomes, roundworms, hookworms, and whipworms – are endemic. The helminthhelminth infectionsinfections Assembly stressed that provision should be made for the regular anthelminthic treatment of school-age children living wherever schistosomes and soil-transmitted nematodes are entrenched. This book emerged from a conference held in Bali under the auspices of the Government of Indonesia and WHO. It reviews the science that underpins the practical approach to helminth control based on deworming. There are articles dealing with the public health significance of helminth infections, with strategies for disease control, and with aspects of anthelminthic chemotherapy using high-quality recommended drugs. Other articles summarize the experience gained in national and local control programmes in countries around the world. Deworming is an affordable, cost-effective public health measure that can be readily integrated with existing health care programmes; as such, it deserves high priority. Sustaining the benefits of deworming depends on having dedicated health professionals, combined with political commitment, community involvement, health education, and investment in sanitation. "Let it be remembered how many lives and what edited by a fearful amount of suffering have been saved by D.W.T. Crompton the knowledge gained of parasitic worms through A. -

208398Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 208398Orig1s000 MICROBIOLOGY/VIROLOGY REVIEW(S) Division of Anti-Infective Products Clinical Microbiology Review NDA 208398 (Original) Page 4 of 59 Table of Contents 1. EXECUTIVE SUMMARY ......................................................................................................................................5 2. INTRODUCTION AND BACKGROUND .............................................................................................................7 2.1. Mebendazole .................................................................................................................................................7 2.2. Biology of roundworm, whipworm and hookworm......................................................................................9 3. NONCLINICAL MICROBIOLOGY STUDIES....................................................................................................11 3.1. Mechanism of action...................................................................................................................................12 3.1.1. Effect on uptake of glucose, amino acids and fatty acids ................................................................12 3.1.2. Effect on morphology......................................................................................................................14 3.2. Activity in vitro/ex vivo..............................................................................................................................16 3.2.1. -

Molecular Epidemiology of Hookworm Infection in the Ashanti Region, Ghana
Yale University EliScholar – A Digital Platform for Scholarly Publishing at Yale Public Health Theses School of Public Health January 2020 Molecular Epidemiology Of Hookworm Infection In The Ashanti Region, Ghana. Emma Allen [email protected] Follow this and additional works at: https://elischolar.library.yale.edu/ysphtdl Recommended Citation Allen, Emma, "Molecular Epidemiology Of Hookworm Infection In The Ashanti Region, Ghana." (2020). Public Health Theses. 1916. https://elischolar.library.yale.edu/ysphtdl/1916 This Open Access Thesis is brought to you for free and open access by the School of Public Health at EliScholar – A Digital Platform for Scholarly Publishing at Yale. It has been accepted for inclusion in Public Health Theses by an authorized administrator of EliScholar – A Digital Platform for Scholarly Publishing at Yale. For more information, please contact [email protected]. Molecular epidemiology of hookworm infection in the Ashanti Region, Ghana. Emma Allen 2020 2020 Master of Public Health Epidemiology of Microbial Diseases Michael Cappello, MD Debbie Humphries, PhD, MPH I. Abstract INTRODUCTION: Parasitic helminth infections have persisted in resource-limited settings around the world, leading to greater numbers of people experiencing sequelae including malnutrition, anemia, and impaired growth and cognitive function among children. Despite periodic deworming efforts, a high burden of disease remains in sub-Saharan Africa. Establishing baseline prevalence of these infections can inform local and national deworming campaign objectives as well as tailor additional interventions accordingly. Here we describe our efforts to determine baseline prevalence and analyze potential risk factors for infection in Ashanti Region, Ghana. OBJECTIVES: The primary objective of this study was to characterize the molecular epidemiology of hookworm and schistosomiasis co-infections among communities surrounding Lake Bosumtwe in Ashanti Region, Ghana. -

Helminths and Helminthiasis
Methodenseminar Helminths and Helminthiasis I. Schabussova (SS 2014) Institute of Specific Prophylaxis and Tropical Medicine Medical University Vienna Introduction and overview • Parasites • Helminths • Developmental stages • Transmission • Life cycle • Localisation • Clinical presentation • Diagnosis • Treatment • Examples Parasite • CDC: A parasite is an organism that lives on • or in a host & gets its food from its host • Schmidt & Roberts (1985): "Parasites are those organisms studied by people who call themselves parasitologists“ • Latin: paras ītus - a person who lives by amusing the rich • Greek: paras ītos – a person who eats at someone else's table CDC: Centers for Disease Control and Prevention Parasite infestation & Parasitosis • Parasite infestation: presence of parasites in/on the host without clinical manifestation • Parasitosis: presence of parasites in/on the host with clinical manifestation (disease) Incubation period & Prepatent period • Incubation period: the period between the infection of an individual by a parasite and the manifestation of the disease it causes • • Prepatent period: the period between infection with a parasite and the demonstration of the parasite in the body - determined by the recovery of an infective form (oocysts, larvae, or eggs) from the blood, urine or feces; is usually shorter than the incubation period Host, Definitive host, Intermediate host & Reservoir • Host: is an organism that harbors a parasite, typically providing nutrition and shelter • Definitive host/primary host : is a host in which the parasite reaches maturity and, if possible, reproduces sexually • Intermediate host/a secondary host: is a host that harbors the parasite only for a short transition period, during which (usually) some developmental stage is completed • Reservoir host: can harbour a pathogen indefinitely with no ill effects.