Hemiptera: Heteroptera)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insect Orders
CMG GardenNotes #313 Insect Orders Outline Anoplura: sucking lice, page 1 Blattaria: cockroaches and woodroaches, page 2 Coleoptera: beetles, page 2 Collembola: springtails, page 4 Dermaptera: earwigs, page 4 Diptera: flies, page 5 Ephemeroptera: mayflies, page 6 Hemiptera (suborder Heteroptera): true bugs, page 7 Hemiptera (suborders Auchenorrhyncha and Sternorrhyncha): aphids, cicadas, leafhoppers, mealybugs, scale and whiteflies, page 8 Hymenoptera: ants, bees, horntails, sawflies, and wasp, page 9 Isoptera: termites, page 11 Lepidoptera: butterflies and moths, page 12 Mallophaga: chewing and biting lice, page 13 Mantodea: mantids, page 14 Neuroptera: antlions, lacewings, snakeflies and dobsonflies, page 14 Odonata: dragonflies and damselflies, page 15 Orthoptera: crickets, grasshoppers, and katydids, page 15 Phasmida: Walking sticks, page 16 Plecoptera: stoneflies, page 16 Psocoptera: Psocids or booklice, page 17 Siphonaptera: Fleas, page 17 Thysanoptera: Thrips, page 17 Trichoptera: Caddisflies, page 18 Zygentomaa: Silverfish and Firebrats, page 18 Anoplura Sucking Lice • Feeds by sucking blood from mammals. • Some species (head lice and crabs lice) feed on humans. Metamorphosis: Simple/Gradual Features: [Figure 1] Figure 1. Sucking lice o Wingless o Mouthparts: Piercing/sucking, designed to feed on blood. o Body: Small head with larger, pear-shaped thorax and nine segmented abdomen. 313-1 Blattaria (Subclass of Dictyoptera) Cockroaches and Woodroaches • Most species are found in warmer subtropical to tropical climates. • The German, Oriental and American cockroach are indoor pests. • Woodroaches live outdoors feeding on decaying bark and other debris. Metamorphosis: Simple/Gradual Figure 2. American cockroach Features: [Figure 2] o Body: Flattened o Antennae: Long, thread-like o Mouthparts: Chewing o Wings: If present, are thickened, semi-transparent with distinct veins and lay flat. -

The Complete Mitochondrial Genome of the Damsel Bug Alloeorhynchus
Int. J. Biol. Sci. 2012, 8 93 Ivyspring International Publisher International Journal of Biological Sciences 2012; 8(1):93-107 Research Paper The Complete Mitochondrial Genome of the Damsel Bug Alloeorhynchus bakeri (Hemiptera: Nabidae) Hu Li1,*, Haiyu Liu1,*, Liangming Cao2, Aimin Shi1, Hailin Yang1, Wanzhi Cai1 1. Department of Entomology, China Agricultural University, Beijing 100193, China 2. The Key Laboratory of Forest Protection of China State Forestry Administration, Research Institute of Forest Ecology, Environment and Protection, Chinese Academy of Forestry, Beijing 100091, China * These authors contributed equally to this work. Corresponding author: Dr. Wanzhi Cai, Department of Entomology, China Agricultural University, Yuanmingyuan West Road, Beijing 100193, China. Phone: 86-10-62732885; Fax: 86-10-62732885; Email: [email protected] © Ivyspring International Publisher. This is an open-access article distributed under the terms of the Creative Commons License (http://creativecommons.org/ licenses/by-nc-nd/3.0/). Reproduction is permitted for personal, noncommercial use, provided that the article is in whole, unmodified, and properly cited. Received: 2011.09.17; Accepted: 2011.11.10; Published: 2011.11.24 Abstract The complete sequence of the mitochondrial DNA (mtDNA) of the damsel bug, Al- loeorhynchus bakeri, has been completed and annotated in this study. It represents the first sequenced mitochondrial genome of heteropteran family Nabidae. The circular genome is 15, 851 bp in length with an A+T content of 73.5%, contains the typical 37 genes that are arranged in the same order as that of the putative ancestor of hexapods. Nucleotide composition and codon usage are similar to other known heteropteran mitochondrial genomes. -

Hemiptera: first Record for an Australian Lophopid (Hemiptera, Lophopidae)
Australian Journal of Entomology (2007) 46, 129–132 Historical use of substrate-borne acoustic production within the Hemiptera: first record for an Australian Lophopid (Hemiptera, Lophopidae) Adeline Soulier-Perkins,1* Jérôme Sueur2 and Hannelore Hoch3 1Muséum National d’Histoire Naturelle, Département Systématique et Évolution, USM 601 MNHN & UMR 5202 CNRS, Case Postale 50, 45, Rue Buffon, F-75005 Paris, France. 2NAMC-CNRS UMR 8620, Bât. 446, Université Paris XI, F-91405 Orsay Cedex, France. 3Museum für Naturkunde, Institut für Systematische Zoologie, Humboldt-Universität zu Berlin Invalidenstr. 43, D- 10115 Berlin, Germany. Abstract Here the first record of communication through substrate-borne vibrations for the Lophopidae family is reported. The signals from Magia subocellata that the authors recorded were short calls with a decreasing frequency modulation. Acoustic vibrations have been observed for other families within the Hemiptera and a scenario concerning the historical use of vibrational communication within the Hemiptera is tested using a phylogenetic inference. The most parsimonious hypothesis suggests that substrate-borne communication is ancestral for the hemipteran order and highlights the groups for which future acoustic research should be undertaken. Key words Cicadomorpha, Coleorrhyncha, evolutionary scenario, Heteroptera, Sternorrhyncha, substrate vibration. INTRODUCTION Lophopidae migrating into America via the Bering land bridge. Some other ancestors of the extant groups moved onto Many animals have been recently recognised for their ability newly emerging land in the Pacific, expanding their distribu- to communicate through substrate-borne vibrations (Hill tion as far east as the Samoan Islands, and as far south as 2001). While elephants produce vibrations transmitted by the Australia (Soulier-Perkins 2000). -

DAMSEL BUGS Hemiptera: Nabidae Nabis Alternatus, N
Modified from Ralph E. Berry. 1998©. Insects and Mites of Economic Importance in the Northwest. 2nd Ed. 221 p. DAMSEL BUGS Hemiptera: Nabidae Nabis alternatus, N. americoferus ___________________________________________________________________________ DESCRIPTION Adults are tan or grayish-brown, with piercing- sucking mouthparts and well-developed wings. The membranous portion of the first pair of wings has a number of small cells around the margin. The front pair of legs is modified for grasping their prey. They have slender bodies, and are about 4 to 12 mm long. Nymphs resemble adults, except they are smaller and have no wings. LIFE HISTORY Western damsel bug adult feeding on lygus Damsel bugs overwinter as adults in protected places around the margins of fields. Adults of the Western damsel bug, N. alternatus, begin emerging in May or early June and N. americolferus begins emerging in late March or early April. Adults begin laying eggs soon after emergence. Eggs are flattened on top and are inserted into soft plant tissue along the stems. Eggs hatch into nymphs, which feed on small insects, mites, or eggs. Early instar nymphs may be found on the soil surface beneath plants and under litter near the stems. N. americolferus has two complete Western Damsel bug nymph generations each year with little overlap between the develoopmental stages. N. alternatus has numerous, overlapping generations during the season. DAMSEL BUG IMPORTANCE ADULTS EGGS, NYMPHS, ADULTS Adults and nymphs feed on many soft-bodied insects, OVERLAPPING GENERATIONS including aphids, spider mites, leafhoppers, small ADULTS caterpillars, and insect eggs including those of the J F M A M J J A S O N D Colorado potato beetle. -

The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V
The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V. Bennett Edwin F. Cook Technical Bulletin 332-1981 Agricultural Experiment Station University of Minnesota St. Paul, Minnesota 55108 CONTENTS PAGE Introduction ...................................3 Key to Adults of Nearctic Families of Semiaquatic Hemiptera ................... 6 Family Saldidae-Shore Bugs ............... 7 Family Mesoveliidae-Water Treaders .......18 Family Hebridae-Velvet Water Bugs .......20 Family Hydrometridae-Marsh Treaders, Water Measurers ...22 Family Veliidae-Small Water striders, Rime bugs ................24 Family Gerridae-Water striders, Pond skaters, Wherry men .....29 Family Ochteridae-Velvety Shore Bugs ....35 Family Gelastocoridae-Toad Bugs ..........36 Literature Cited ..............................37 Figures ......................................44 Maps .........................................55 Index to Scientific Names ....................59 Acknowledgement Sincere appreciation is expressed to the following individuals: R. T. Schuh, for being extremely helpful in reviewing the section on Saldidae, lending specimens, and allowing use of his illustrations of Saldidae; C. L. Smith for reading the section on Veliidae, checking identifications, and advising on problems in the taxon omy ofthe Veliidae; D. M. Calabrese, for reviewing the section on the Gerridae and making helpful sugges tions; J. T. Polhemus, for advising on taxonomic prob lems and checking identifications for several families; C. W. Schaefer, for providing advice and editorial com ment; Y. A. Popov, for sending a copy ofhis book on the Nepomorpha; and M. C. Parsons, for supplying its English translation. The University of Minnesota, including the Agricultural Experi ment Station, is committed to the policy that all persons shall have equal access to its programs, facilities, and employment without regard to race, creed, color, sex, national origin, or handicap. The information given in this publication is for educational purposes only. -

Review of the West Indian Arachnocoris Scott, 1881 (Hemiptera: Nabidae), with Descriptions of Two New Species, and a Catalog of the Species1
Life: The Excitement of Biology 4(1) 32 Review of the West Indian Arachnocoris Scott, 1881 (Hemiptera: Nabidae), with Descriptions of Two New Species, and a Catalog of the Species1 Javier E. Mercado2, Jorge A. Santiago-Blay3, and Michael D. Webb4 Abstract: We review the West Indian species of Arachnocoris, a genus of spider-web dwelling kleptoparasitic nabids. We recognize five species: A. berytoides Uhler from Grenada, A. darlingtoni n. sp. from Hispaniola, A. karukerae Lopez-Moncet from Guadeloupe, A. portoricensis n. sp. from Puerto Rico, and A. trinitatis Bergroth from Trinidad. West Indian Arachnocoris antennal and profemoral color banding patterns are useful diagnostic characters and may have evolved to mimic their spider hosts, which are often island endemic spiders in the family Pholcidae. We provide a simplified and illustrated key to the species based on external characters. A catalog for the 16 recognized species of Arachnocoris is presented. Keywords: Hemiptera, Nabidae, Arachnocoris, new species, Neotropical, West Indies Introduction The Nabidae are a relatively small family in the insect order Hemiptera with approximately 20-30 genera and 400-500 described species (Henry 2009, Faúndez and Carvajal 2014). All described species are terrestrial predators. Some species are considered beneficial to humans as these help control populations of agricultural pests. Several species of Nabis have been reported as biting humans (Faúndez 2015). Within the Nabidae Arachnocoris is one of two genera in the tribe Arachnocorini. The arachnophilic genus5 Arachnocoris Scott is a small and little-known group of specialized kleptoparasitic nabids that spend their life- stages living in a relatively treacherous habitat, namely a spider’s web, particularly non-sticky portions of it (Henry 1999; Mercado-Santiago-Blay 2015; Figure 1, this paper). -
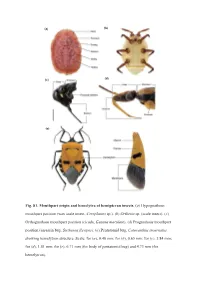
Page 1 (A) (E) Fig. S1. Mouthpart Origin and Hemelytra of Hemipteran
(a) (b) (d) (c) (e) Fig 61. Mouthpart origin and hemelytra of hemipteran insects. (a) Hypognathous mouthpart position (wax scale insect, Ceroplastes sp.). (b) Orthezia sp. (scale insect). (c) Orthognathous mouthpart position (cicada, Gaeana maculate). (d) Prognathous mouthpart position (assassin bug, Sirthenea flavipes). (e) Pentatomid bug, Catacanthus incarnatus showing hemelytron structure. Scale: for (a), 0.40 mm; for (b), 0.65 mm; for (c), 3.84 mm; for (d), 1.81 mm; for (e), 6.71 mm (for body of pentatomid bug) and 4.73 mm (for hemelytron). Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG1 PCG2 Sternorrhyncha Cicadomorpha Fulgoromorpha Coleorrhyncha Heteroptera PCG3 RNA )LJ62. AliGROOVE analysis for codon positions of protein-coding genes (PCGs) and RNA genes. PCG1, the first codon position of PCGs. PCG2, the second codon position of PCGs. PCG3, the third codon position of PCGs. RNA, sequences of tRNA and rRNA genes. The mean similarity score between sequences is represented by a colored square, based on AliGROOVE scores from -1, indicating great difference in rates from the remainder of the data set, that is, heterogeneity (red coloring), to +1, indicating that ratesmatch all other comparisons (blue coloring). Bactericera sinica Sternorrhyncha Cicadomorpha Coleorrhyncha Fulgoromorpha Dipsocoromorpha Gerromorpha Enicocephalomorpha Nepomorpha Leptopodomorpha Cimicomorpha Heteroptera Pentatomomorpha Eusthenes cupreus FigS33K\ORJHQHWLFWUHHLQIHUUHGIURP3K\OR%D\HVDQDO\VLVRIWKH3&*51$GDWDVHWXQGHUWKe &$7*75PL[WXUHPRGHO9DOXHVDWQRGHVDUH%D\HVLDQ33V -

First Ever Report of a Bite by Nabis Argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) on a Human
Short Communication Biodiversity and Natural History (2017) Vol. 3, No. 2, 45-47 First ever report of a bite by Nabis argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) on a human Primer registro de una picadura de Nabis argentinus Meyer-Dür (Hemiptera: Heteroptera: Nabidae) en un ser humano Marcela Cornelis1, Fernando Diez1 & María del Carmen Coscarón2 1Universidad Nacional de La Pampa, Facultad de Ciencias Exactas y Naturales, Uruguay 151 L6300CLB, Santa Rosa, La Pampa, Argentina. 2Universidad Nacional de La Plata, Facultad de Ciencias Naturales y Museo, División Entomología, Paseo del Bosque s/n 1900, La Plata, Buenos Aires, Argentina. *Correspondence author: [email protected] Abstract Herein, we described the first ever reported bite of Nabis argentinus Meyer-Dür 1870 on a human. The bite was registered in the locality Santa Rosa La Pampa, Argentina (36°37'29.02"S, 64°17'19.13"W). The insect was not provoked by the victim, and thus, the bite was probably not in self-defense. We therefore concluded that the insect bite the victim because it was searching for sources of hydration. Key words: adventitious bite, nabids, predaceous habit, record, symptomatology. Resumen Se describe el primer caso de una picadura adventicia en un ser humano por Nabis argentinus Meyer-Dür 1870, en la localidad de Santa Rosa La Pampa, Argentina (36°37'29.02"S, 64°17'19.13"W). El insecto no fue provocado por la víctima, por lo que probablemente la picadura no fue en defensa propia. Por lo tanto, concluimos que el insecto picó a la víctima en búsqueda de recursos de hidratación. -

A Faunistic Approach to Assess Potential Side-Effects of Genetically Modified Bt-Corn on Non-Target Arthropods Under Field Conditions
Biocontrol Science and Technology (March 2004), Vol. 14, No. 2, 129Á/170 A Faunistic Approach to Assess Potential Side-Effects of Genetically Modified Bt-Corn on Non-Target Arthropods Under Field Conditions 1 2 1 1 M. P. CANDOLFI ,K.BROWN, C. GRIMM , B. REBER AND H. SCHMIDLI1 1Syngenta Crop Protection AG, CH-4002 Basel, Switzerland; 2Ecotox Limited, Tavistock, Devon, UK (Received 31 October 2001; accepted 13 May 2003) A faunistic study investigating the potential side-effects of corn (Zea mays) genetically modified to express a truncated Cry1Ab protein derived from Bacillus thuringiensis subsp. kurstaki, on non-target arthropods was carried out under field conditions. The communities of non-target arthropods in the soil, on the leaves and flying in the crop area were monitored throughout the growing season. Water-treated, untransformed corn served as a control, and a spray application of a bacterial Bt insecticide (Delfin WG) and a synthetic insecticide (Karate Xpress) used to control the European corn borer (Ostrinia nubilalis; Lepidoptera: Pyralidae) acted as positive reference treatments. Results were analyzed using a principal response curve. Significantly lower infestations by the lepidopteran target species O. nubilalis were observed in the Bt-corn plots compared to the control. No effects of Bt-corn on the communities of soil dwelling and non-target plant dwelling arthropods were observed. A trend towards a community effect on flying arthropods was observed with lower abundance of adult Lepidoptera, flies in the families Lonchopteridae, Mycetophilidae and Syrphidae, and the hymenopteran parasitoids Ceraphronidae. Effects were weak and restricted to two sampling dates corresponding to anthesis. -

Building-Up of a DNA Barcode Library for True Bugs (Insecta: Hemiptera: Heteroptera) of Germany Reveals Taxonomic Uncertainties and Surprises
Building-Up of a DNA Barcode Library for True Bugs (Insecta: Hemiptera: Heteroptera) of Germany Reveals Taxonomic Uncertainties and Surprises Michael J. Raupach1*, Lars Hendrich2*, Stefan M. Ku¨ chler3, Fabian Deister1,Je´rome Morinie`re4, Martin M. Gossner5 1 Molecular Taxonomy of Marine Organisms, German Center of Marine Biodiversity (DZMB), Senckenberg am Meer, Wilhelmshaven, Germany, 2 Sektion Insecta varia, Bavarian State Collection of Zoology (SNSB – ZSM), Mu¨nchen, Germany, 3 Department of Animal Ecology II, University of Bayreuth, Bayreuth, Germany, 4 Taxonomic coordinator – Barcoding Fauna Bavarica, Bavarian State Collection of Zoology (SNSB – ZSM), Mu¨nchen, Germany, 5 Terrestrial Ecology Research Group, Department of Ecology and Ecosystem Management, Technische Universita¨tMu¨nchen, Freising-Weihenstephan, Germany Abstract During the last few years, DNA barcoding has become an efficient method for the identification of species. In the case of insects, most published DNA barcoding studies focus on species of the Ephemeroptera, Trichoptera, Hymenoptera and especially Lepidoptera. In this study we test the efficiency of DNA barcoding for true bugs (Hemiptera: Heteroptera), an ecological and economical highly important as well as morphologically diverse insect taxon. As part of our study we analyzed DNA barcodes for 1742 specimens of 457 species, comprising 39 families of the Heteroptera. We found low nucleotide distances with a minimum pairwise K2P distance ,2.2% within 21 species pairs (39 species). For ten of these species pairs (18 species), minimum pairwise distances were zero. In contrast to this, deep intraspecific sequence divergences with maximum pairwise distances .2.2% were detected for 16 traditionally recognized and valid species. With a successful identification rate of 91.5% (418 species) our study emphasizes the use of DNA barcodes for the identification of true bugs and represents an important step in building-up a comprehensive barcode library for true bugs in Germany and Central Europe as well. -

Vol. 14, No. 1 Spring 1981 the GREAT LAKES ENTOMOLOGIST Published by the Michigan Entomological Society Volume 14 No
The GREAT LAKES ENTOMOLOGIST Vol. 14, No. 1 Spring 1981 THE GREAT LAKES ENTOMOLOGIST Published by the Michigan Entomological Society Volume 14 No. 1 ISSN 0090-0222 TABLE OF CONTENTS Annotated List of Indiana Scolytidae (Coleoptera) Mark Deyrup .................................................. Seasonal Flight Patterns of Hemiptera in a North Carolina Black Walnut Plantation. 2. Coreoida J. E. McPherson and B. C. Weber .......................................... 11 Seasonal Flight Patterns of Hemiptera in a North Carolina Black Walnut Plantation. 3. Reduvioidea J. E. McPherson and B. C. Weber .......................................... 15 Seasonal Flight Patterns of Hemiptera in a North Carolina Black Walnut Plantation. 4. Cimicoidea J. E. McPherson and B. C. Weber .......................................... 19 Fourlined Plant Bug (Hemiptera: Miridae), A Reappraisal: Life History, Host Plants, and Plant Response to Feeding A. G. Wheeler, Jr. and Gary L. Miller.. ..................................... 23 Hawthorn Lace Bug (Hemiptera: Tingidae), First Record of Injury to Roses, with a Review of Host Plants A. G. Wheeler, Jr. ........................................................ 37 Notes on the Biology of Nersia florens (Homoptera: Fulgoroidea: Dictyopharidae) with Descriptions of Eggs, and First, Second, and Fifth Instars S. W. Wilson and J. E. McPherson.. ...................... Ontogeny of the Tibial Spur in Megamelus davisi (Homoptera: Delphacidae) and its Bearing on Delphacid Classification S. W. Wilson and J. E. McPherson.. ..................... -

Biology of Natural Enemies at Greenhouse Scale
SESSI O N III BI O L O G Y O F N A T UR A L E N E M I ES A T G R E E N H O USE SC A L E 41! ! 42! SESSION III Use of the predators Orius laevigatus and Aeolothrips spp. to control F rankliniella occidentalis populations in greenhouse peppers in the region of Monastir, Tunisia Mohamed Elimem, Brahim Chermiti /DERUDWRLUHG¶HQWRPRORJLHHWGHOXWWHELRORJLTXH,QVWLWXW6XSpULHXUAgronomique de Chott- Mériem 4042, Université de Sousse, Tunisia The use of Orius laevigatus and Aeolothrips spp. to control Frankliniella occidentalis in greenhouse peppers in Tunisia produced different results according to predator species and different doses and release frequencies. In the case of the predator bug O. laevigatus, the most effective dose was 1 individual per m! repeated three times with an interval of one week. Although O. laevigatus releases caused F. occidentalis populations to decrease for only one week, the predator was able to become installed in the crop and to proliferate. In the case of the predatory thrips Aeolothrips spp., no individuals were recorded in the pepper greenhouse even after the third release. However, the F. occidentalis population decreased to low average values of 0.42 and 0.06 thrips per flower as a result of the spontaneous colonization of individuals of O. laevigatus from other greenhouses. 43! SESSION III Evaluation of four lacewing species for aphid control in sweet pepper Gerben Messelink1, Chantal Bloemhard1, Hans Hoogerbrugge2, Jeroen van Schelt2 1Wageningen UR Greenhouse Horticulture, PO Box 20, 2265 ZG Bleiswijk, The Netherlands; 2Koppert Biological Systems, PO Box 155, 2650 AD Berkel & Rodenrijs, The Netherlands Four species of lacewings were evaluated for control of the peach aphid Myzus persicae in sweet pepper.