The Impact of Herbivorous Insects on Australian Rainforest Tree Canopies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A4 Template with Cover and Following Page
Powerful Owl Project Update – December 2015 Caroline Wilson, Holly Parsons & Janelle Thomas Thank-you to all of you for being involved with another successful year of the Powerful Owl Project. We had some changes this year, with our main grant funding finishing Caroline Wilson and Janelle Thomas from the Threatened Bird Network (TBN) and Holly Parsons from the Birds in Backyards Program took over the running of the project for BirdLife Australia. Generous donations from the NSW Twitch-a-thon has allowed us to complete this year’s research and allows us to continue in 2016. This season we have had over 120 registered volunteers involved, including over 50 new volunteers who were recruited to the project early this year. You have helped us monitor over 80 Powerful Owl breeding sites, allowing the project to cover a lot of ground throughout Greater Sydney, the NSW Central Coast and Newcastle. The data you have collected is really important for the effective management of urban Powerful Owl populations and this information is shared with land mangers and local councils. So thank-you! We really appreciate the amazing work carried out by our volunteers; with your help we have learned so much about these birds, and this information is helping us protect this unique and amazing species. Read on to hear about all we have achieved in 2015. Powerful Owl chick from 2015, peeking out of the hollow (taken by Christine Melrose) March 2015 workshop The March workshop was held to train our new volunteers, update everyone on the findings from the project and to say thank-you to our existing volunteers – some of which have been with us since 2011. -

The Wood Cross Sections of Hermann Nördlinger (1818–1897)
IAWA Journal, Vol. 29 (4), 2008: 439–457 THE WOOD CROSS SECTIONS OF HERMANN NÖRDLINGER (1818–1897) Ben Bubner Leibniz-Zentrum für Agrarlandschaftsforschung (ZALF) e.V., Institut für Landschaftsstoffdynamik, Eberswalder Str. 84, 15374 Müncheberg, Germany [E-mail: [email protected]] SUMMARY Hermann Nördlinger (1818–1897), forestry professor in Hohenheim, Germany, published a series of wood cross sections in the years 1852 to 1888 that are introduced here to the modern wood anatomist. The sec- tions, which vary from 50 to 100 μm in thickness, are mounted on sheets of paper and their quality is high enough to observe microscopic details. Their technical perfection is as remarkable as the mode of distribution: sections of 100 wood species were presented in a box together with a booklet containing wood anatomical descriptions. These boxes were dis- tributed as books by the publisher Cotta, from Stuttgart, Germany, with a maximum circulation of 500 per volume. Eleven volumes comprise 1100 wood species from all over the world. These include not only conifers and broadleaved trees but also shrubs, ferns and palms representing a wide variety of woody structures. Excerpts of this collection were also pub- lished in Russian, English and French. Today, volumes of Nördlingerʼs cross sections are found in libraries throughout Europe and the United States. Thus, they are relatively easily accessible to wood anatomists who are interested in historic wood sections. A checklist with the content of each volume is appended. Key words: Cross section, wood collection, wood anatomy, history. INTRODUCTION Wood scientists who want to distinguish wood species anatomically rely on thin sec- tions mounted on glass slides and descriptions in books that are illustrated with micro- photographs. -
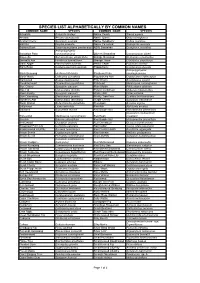
Species List Alphabetically by Common Names
SPECIES LIST ALPHABETICALLY BY COMMON NAMES COMMON NAME SPECIES COMMON NAME SPECIES Actephila Actephila lindleyi Native Peach Trema aspera Ancana Ancana stenopetala Native Quince Guioa semiglauca Austral Cherry Syzygium australe Native Raspberry Rubus rosifolius Ball Nut Floydia praealta Native Tamarind Diploglottis australis Banana Bush Tabernaemontana pandacaqui NSW Sassafras Doryphora sassafras Archontophoenix Bangalow Palm cunninghamiana Oliver's Sassafras Cinnamomum oliveri Bauerella Sarcomelicope simplicifolia Orange Boxwood Denhamia celastroides Bennetts Ash Flindersia bennettiana Orange Thorn Citriobatus pauciflorus Black Apple Planchonella australis Pencil Cedar Polyscias murrayi Black Bean Castanospermum australe Pepperberry Cryptocarya obovata Archontophoenix Black Booyong Heritiera trifoliolata Picabeen Palm cunninghamiana Black Wattle Callicoma serratifolia Pigeonberry Ash Cryptocarya erythroxylon Blackwood Acacia melanoxylon Pink Cherry Austrobuxus swainii Bleeding Heart Omalanthus populifolius Pinkheart Medicosma cunninghamii Blue Cherry Syzygium oleosum Plum Myrtle Pilidiostigma glabrum Blue Fig Elaeocarpus grandis Poison Corkwood Duboisia myoporoides Blue Lillypilly Syzygium oleosum Prickly Ash Orites excelsa Blue Quandong Elaeocarpus grandis Prickly Tree Fern Cyathea leichhardtiana Blueberry Ash Elaeocarpus reticulatus Purple Cherry Syzygium crebrinerve Blush Walnut Beilschmiedia obtusifolia Red Apple Acmena ingens Bollywood Litsea reticulata Red Ash Alphitonia excelsa Bolwarra Eupomatia laurina Red Bauple Nut Hicksbeachia -

Birdlife Northern NSW Autumn Campout 2021 DORRIGO Friday 12Th to Sunday 14Th September at Dorrigo Mountain Holiday Park, 3991 Waterfall Way, Ph (02) 6657 2564; Email
BirdLife Northern NSW Autumn Campout 2021 DORRIGO Friday 12th to Sunday 14th September at Dorrigo Mountain Holiday Park, 3991 Waterfall Way, ph (02) 6657 2564; email PROGRAM and OUTINGS (subject to changes/cancellation to comply with NSW COVID public health orders at the time) Friday 12th registration (required for our legal obligations); (TBC) optional guided Night Walk in Dorrigo NP; the evening meal is your own arrangement 3.00 - 5.00pm register in the Meeting Hall at Dorrigo Mountain Holiday Park, near the Park Office (please observe COVID public health orders) - confirm contact details, check outing details, enter 'Final Bird Count', buy raffle tickets, Night Walk payments - $20 (cash only please) TBC: 6.30 - 8.30pm ranger-guided Night Walk (limit 20 people) in Dorrigo National Park, enter your interest on the registration form; payment $20 cash on Friday 12 March. - free choice for Fri & Sat night meals e.g. self-catering in cabins, or use communal kitchen & BBQ facilities at the campground. In Dorrigo town centre: the usual take- away, pub dinner at Dorrigo Heritage Hotel, Chinese at RSL Club (6-8pm), North Dorrigo Restaurant (Fri nights only by booking ph:(02 6657 5150); Bellingen has many eateries (30min drive down Waterfall Way). For Friday or Monday we recommend Canopy Cafe in the pleasant setting of Dorrigo National Park (open 9-4.30). Saturday 5th guided daytime Outings see page 2 (COVID public health orders must be observed) NB: there is no group dinner for this campout, see Friday dining suggestions Sunday 6th guided daytime Outings and 5.00pm for final Bird Call byo drinks & snacks in the Meeting Hall, Dorrigo Mountain Holiday Park (COVID number limit may apply) Saturday 5th and Sunday 6th Outings Program page 2 NOTE your departure time & meeting location - please be on time, your group may not be able to wait Because environmental conditions or leaders may change, or new information is received, routes could be modified by guides on the day for safety reasons and to maximise bird-watching opportunities along each route. -

(Phascolarctos Cinereus) on the North Coast of New South Wales
A Blueprint for a Comprehensive Reserve System for Koalas (Phascolarctos cinereus) on the North Coast of New South Wales Ashley Love (President, NPA Coffs Harbour Branch) & Dr. Oisín Sweeney (Science Officer, NPA NSW) April 2015 1 Acknowledgements This proposal incorporates material that has been the subject of years of work by various individuals and organisations on the NSW north coast, including the Bellengen Environment Centre; the Clarence Environment Centre; the Nambucca Valley Conservation Association Inc., the North Coast Environment Council and the North East Forest Alliance. 2 Traditional owners The NPA acknowledges the traditional Aboriginal owners and original custodians of the land mentioned in this proposal. The proposal seeks to protect country in the tribal lands of the Bundjalung, Gumbainggir, Dainggatti, Biripi and Worimi people. Citation This document should be cited as follows: Love, Ashley & Sweeney, Oisín F. 2015. A Blueprint for a comprehensive reserve system for koalas (Phascolarctos cinereus) on the North Coast of New South Wales. National Parks Association of New South Wales, Sydney. 3 Table of Contents Acknowledgements ....................................................................................................................................... 2 Traditional owners ........................................................................................................................................ 3 Citation ......................................................................................................................................................... -

Reconstructing the Basal Angiosperm Phylogeny: Evaluating Information Content of Mitochondrial Genes
55 (4) • November 2006: 837–856 Qiu & al. • Basal angiosperm phylogeny Reconstructing the basal angiosperm phylogeny: evaluating information content of mitochondrial genes Yin-Long Qiu1, Libo Li, Tory A. Hendry, Ruiqi Li, David W. Taylor, Michael J. Issa, Alexander J. Ronen, Mona L. Vekaria & Adam M. White 1Department of Ecology & Evolutionary Biology, The University Herbarium, University of Michigan, Ann Arbor, Michigan 48109-1048, U.S.A. [email protected] (author for correspondence). Three mitochondrial (atp1, matR, nad5), four chloroplast (atpB, matK, rbcL, rpoC2), and one nuclear (18S) genes from 162 seed plants, representing all major lineages of gymnosperms and angiosperms, were analyzed together in a supermatrix or in various partitions using likelihood and parsimony methods. The results show that Amborella + Nymphaeales together constitute the first diverging lineage of angiosperms, and that the topology of Amborella alone being sister to all other angiosperms likely represents a local long branch attrac- tion artifact. The monophyly of magnoliids, as well as sister relationships between Magnoliales and Laurales, and between Canellales and Piperales, are all strongly supported. The sister relationship to eudicots of Ceratophyllum is not strongly supported by this study; instead a placement of the genus with Chloranthaceae receives moderate support in the mitochondrial gene analyses. Relationships among magnoliids, monocots, and eudicots remain unresolved. Direct comparisons of analytic results from several data partitions with or without RNA editing sites show that in multigene analyses, RNA editing has no effect on well supported rela- tionships, but minor effect on weakly supported ones. Finally, comparisons of results from separate analyses of mitochondrial and chloroplast genes demonstrate that mitochondrial genes, with overall slower rates of sub- stitution than chloroplast genes, are informative phylogenetic markers, and are particularly suitable for resolv- ing deep relationships. -

Fauna Survey, Wingham Management Area, Port Macquarie Region
This document has been scanned from hard-copy archives for research and study purposes. Please note not all information may be current. We have tried, in preparing this copy, to make the content accessible to the widest possible audience but in some cases we recognise that the automatic text recognition maybe inadequate and we apologise in advance for any inconvenience this may cause. FOREST RESOURCES SERIES NO. 19 FAUNA SURVEY, WINGHAM MANAGEMENT AREA, PORT MACQUARIE REGION PART 1. MAMMALS BY ALAN YORK \ \ FORESTRY COMMISSION OF NEW SOUTH WALES ----------------------------- FAUNA SURVEY, WINGHAM MANAGEMENT AREA, PORT MACQUARIE REGION PART 1. MAMMALS by ALAN YORK FOREST ECOLOGY SECTION WOOD TECHNOLOGY AND FOREST RESEARCH DIVISION FORESTRY COMMISSION OF NEW SOUTH WALES SYDNEY 1992 Forest Resources Series No. 19 March 1992 The Author: AIan York, BSc.(Hons.) PhD., Wildlife Ecologist, Forest Ecology Section, Wood Technology and Forest Research Division, Forestry Commission ofNew South Wales. Published by: Forestry Commission ofNew South Wales, Wood Technology and Forest Research Division, 27 Oratava Avenue, West Pennant Hills, 2125 P.O. Box lOO, Beecroft 2119 Australia. Copyright © 1992 by Forestry Commission ofNew South Wales ODC 156.2:149 (944) ISSN 1033-1220 ISBN 07305 5663 8 Fauna Survey, Wingham Management Area, -i- PortMacquarie Region Part 1. Mammals TABLE OF CONTENTS PAGE INTRODUCTION 1 1. The Wingham Management Area 1 (a) Location 1 (b) Physical environment 3 (c) Vegetation communities 3 (d) Fire 5 (e) Timber harvesting : 5 SURVEY METHODOLOGy 7 1. Overall Sampling Strategy 7 (a) General survey 7 (b) Plot-based survey 7 (i) Stratification 1:Jy Broad Forest Type : 8 (ii) Stratification by Altitude 8 (iii) Stratification 1:Jy Management History 8 (iv) Plot selection 9 (v) Special considerations 9 (vi) Plot establishment 10 (vii) Plot design........................................................................................................... -

Eidothea Hardeniana (Nightcap Oak) September 2004 © Department of Environment and Conservation (NSW), July 2004
Approved NSW & National Recovery Plan Eidothea hardeniana (Nightcap Oak) September 2004 © Department of Environment and Conservation (NSW), July 2004. This work is copyright. However, material presented in this plan may be copied for personal use or published for educational purposes, providing that any extracts are fully acknowledged. Apart from this and any other use as permitted under the Copyright Act 1968, no part may be reproduced without prior written permission from NSW Department of Environment and Conservation. NSW Department of Environment and Conservation 43 Bridge Street (PO Box 1967) Hurstville NSW 2220 Tel: 02 9585 6444 www.nationalparks.nsw.gov.au Requests for information or comments regarding the recovery program for the Nightcap Oak are best directed to: The Nightcap Oak Recovery Co-ordinator Threatened Species Unit, North East Branch NSW Department of Environment and Conservation Locked Bag 914 Coffs Harbour NSW 2450 Tel: 02 6651 5946 Cover illustrator: Lesley Elkan © Botanic Gardens Trust, Sydney Cover illustration: Adult and juvenile leaves and fruit of Eidothea hardeniana This plan should be cited as follows: NSW Department of Environment and Conservation 2004, Recovery Plan for the Nightcap Oak (Eidothea hardeniana), Department of Environment and Conservation (NSW), Hurstville. ISBN 0 7313 6781 2 Recovery Plan The Nightcap Oak Draft Recovery Plan The Tumut Grevillea Recovery Plan for the Nightcap Oak (Eidothea hardeniana) Foreword The New South Wales Government established a new environment agency on 24 September 2003, the Department of Environment and Conservation (NSW), which incorporates the New South Wales National Parks and Wildlife Service. Responsibility for the preparation of Recovery Plans now rests with this new department. -

RIVERDENE TUBESTOCK (50X50x150mm)
RIVERDENE TUBESTOCK (50x50x150mm) KEY : B= Bushtucker G= Grass F = Fodder A = Aquatic T = Timber Production C = Groundcover O = Ornamental (non Native) FN – Fern V – Vine/Climber NAME COMMON NAME COMMENT sandstone areas of the Bulga & Putty districts. Frost & sweetly scented yellow flowers. Grows to 1.5m. Abrophyllum ornans - Native Hydrangea- Tall shrub or drought hardy. Responds well to regular pruning. small tree from 3-6m high. Attractive bushy shrub, best Acacia buxifolia - Box Leaf Wattle - Evergreen shrub to B Acacia decurrens - Green Wattle - A fast growing small in a cool moist position in well drained soils. Ideal with 2m, blue green foliage and massed golden yellow to intermediate spreading tree with attractive dark green ferns. Flowers yellowish white & fragrant. Hardy to light flowers. Best in well drained soils but will withstand short fern-like foliage, & large racemes of yellow ball-flowers in drought only. periods of waterlogging. Full or part shade. Winter. Acacia amblygona - Fan Wattle - Small, spreading shrub Acacia concurrens –Curracabah - Shrub or small tree to Acacia doratoxylon – Currawong - Tall shrub or small ranging from completely prostrate in habit to about 1.5 8m high. Rod like flowers, bright yellow in spring. Very tree up to 8 meters high. Best in well drained soil in full metres high. It has bright yellow flowers over winter and hardy & useful small shade tree. Best in full sun & well sun or dappled shade. Useful forage for farm stock. spring. Likes well drained soils and sunny aspect. drained soil. Frost hardy. Hardy to frost and drought when established. Acacia barringtonensis – Barrington - Decorative shrub Acacia coriacea – Wirewood - Tall shrub 4-5m high. -

Guy Fawkes River National Park Horse Management Plan
NORTHERN BRANCH Guy Fawkes River National Park Horse Management Plan For further information about this plan please contact: The Manager, Dorrigo Plateau Area PO Box 170 Dorrigo NSW 2453 ph: 02 6657 2309 fax: 02 6657 2145 Main cover image: View of Guy Fawkes River National Park (Sean Leathers © DEC) Inset images (from left): - Mare and foal (Brad Nesbitt © DEC) - Trap at Wonga Flats in operation (Brad Nesbitt © DEC) This publication should be cited as: NSW National Parks and Wildlife Service (2006) Guy Fawkes River National Park: Horse Management Plan. Department of Environment and Conservation NSW, Sydney South. Published by Department of Environment and Conservation NSW 59–61 Goulburn Street PO Box A290, Sydney South NSW 1232 Ph: 02 9995 5000 (switchboard) Ph: 1300 361 967 (national parks information and publications requests) Fax: 02 9995 5999 TTY: 02 9211 4723 [email protected] Website: www.environment.nsw.gov.au ISBN: 1 74137 976 8 Copyright © Department of Environment and Conservation NSW Material presented in the report and bibliography can be copied for personal use or published for non- commercial purposes, provided that copyright is fully acknowledged. July 2006 DEC 2006/391 Executive Summary The Guy Fawkes River National Park is regarded as a “biodiversity hotspot” with over 40 different vegetation communities, 28 threatened plant species, 24 threatened fauna species and significant areas of old growth forest protected within the reserve. It contains spectacular examples of valley and rugged river gorges including the deeply incised Guy Fawkes River Valley and the rugged gorges of the Aberfoyle, Sara and Henry Rivers. -

Introduction the Need for New Reserves
Koala Strategy Submissions, PO Box A290, Sydney South NSW 1232, [email protected] CC. [email protected] 2nd March 2017. NPA SUBMISSION: WHOLE OF GOVERNMENT KOALA STRATEGY; SAVING OUR SPECIES DRAFT STRATEGY AND REVIEW OF STATE ENVIRONMENT PLANNING POLICY 44—KOALA HABITAT PROTECTION Introduction The National Parks Association of NSW (NPA), established in 1957, is a community-based organisation with over 20,000 supporters from rural, remote and urban areas across the state. NPA promotes nature conservation and evidence- based natural resource management. We have a particular interest in the protection of the State’s biodiversity and supporting ecological processes, both within and outside of the formal conservation reserve system. NPA has a long history of engagement with both government and non-government organisations on issues of park management. NPA appreciates the opportunity to comment on the whole of government koala strategy (the strategy), the Saving Our Species iconic koala project (the SOS project) and the Explanation of intended effect: State Environment and Planning Policy 44 (Koala Habitat Protection) (SEPP 44). Please note, NPA made a submission on SEPP 44 in late 2016 prior to the deadline being extended which we have reattached at the end of this document (Appendix 1). NPA was also consulted on the SOS programme in August 2016, which we greatly appreciated. We provided OEH with feedback on the programme at that time, which also contained an exploration of issues facing koalas, which we have reattached in Appendix 2. NPA has had significant input to the development of the Stand Up For Nature (SUFN) submission to this consultation. -

Associations of Societies for Growing Australian Plants
Page 1 Associations of Societies for Growing Australian Plants – Rainforest Study Group – No.62 (7) June 2006 Associations of Societies for Growing Australian Plants ASGAP Rainforest Study Group NEWSLETTER No 62. (7) June 2006 ISSN 0729-5413 Annual Subscription $5, $10 overseas Photos: www.web-a-file.com Study Group Webpage (under construction): http://farrer.csu.edu.au/ASGAP/rainfor.html Email: [email protected] Address: Kris Kupsch, 28 Plumtree Pocket, Burringbar, Australia, 2483. Ph. (02) 66771466 Mob. 0439557438 Introduction ASGAP trip to Sydney Nov 2005 It has been a long while since I wrote a During my brief visit to Sydney in November newsletter, I apologise for taking so long. last year as part of an invitation to speak at a Since the last newsletter the family and I have SGAP meeting in Ermington, I got to do the moved back to the Wet Tropics. I now work following: as an Environmental Scientist undertaking 1. I was escorted by Cas Liber, ASGAP vegetation surveys and compiling Banksia Study Group leader. Cas environmental management plans for parts of toured me through the Botanic the Wet Tropics World Heritage Area. This Gardens, his garden, among others. has been a rather large transition, leaving Many thanks to Cas and his family. behind my garden and all of my immediate 2. I visited Betty Rymers garden at plans in NSW; the job was too good to refuse. Kenthurst. Betty has a notable I wish everyone the best with their rainforest garden including a large endeavours and hope this newsletter was Brachychiton discolor, Dianella worth the wait.