Euromeeting Amsterdam 2013
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Horsham Township Council
Horsham Township PRSRT STD 1025 Horsham Road U.S. POSTAGE Horsham, PA 19044 PAID HARRISBURG, PA PERMIT NO. 609 Horsham Township Report FALL 2015 Newsletter • www.horsham.org • Published by Horsham Township Council Township Council Authorizes Review of Township Building orsham Township Council has authorized a Hrequest for proposals from architectural firms to conduct a feasibility study of the existing municipal building. The study will explore a variety of scenarios FALL 2015 Newsletter to meet the long-term facility needs. Recently, William Walker, Township Manager gave This Community Newsletter is produced for Horsham Township Council a presentation on the condition and ® hometownpress by Hometown Press • 215.257.1500 • All rights reserved This newsletter is published challenges of the existing building. The building was To Place An Ad Call Rosemary • 215-805-2121 by the Horsham Township built in three phases with the middle section built in Council to provide residents 1959, the east wing built in 1971 and the west wing with important information built in 1989. Each addition was added to meet the about their township. Township’s growing demands. Before the Police Department moved into their new building in December 2009, 64 full-time and 10 part-time TOWNSHIP MANAGER employees worked in the Township Building. With the Police relocated on the complex and William T. Walker ACE MOVING SERVICES Recreation Services now at the Library, there are 14 full-time and two part timers working in the BOARD MEETINGS building. 7.5” width x 1.75” height Township Council Due to the age of the building there are some major challenges that include a leaking roof, two 1st & 4th Monday @ 7:00PM roof mounted air conditioning units that no longer work, water infiltration into the lower level, Need artwork 2nd Wednesday @ 8:00PM deterioration of the building façade and concrete, ADA issues, security issues, old electrical fixtures Planning Commission that bulbs are no longer made for and technology that is not up to current standards. -

Vendor Amount Paid 003851 MALPHRUS CONSTR.CO. Total
Beaufort County Vendors Paid Fiscal Year 2007 Vendor Amount Paid 003851 MALPHRUS CONSTR.CO. Total 16,361,272.79 001166 BLUE CROSS AND BLUE Total 3,744,128.99 008161 REA CONTRACTING, LLC Total 3,172,453.95 000213 HICKORY HILL LANDFIL Total 2,936,246.25 005050 SC COUNTIES WORKERS Total 2,886,871.00 009131 UNICARE LIFE & HEALT Total 2,552,661.34 002374 BEAUFORT CONSTRUCTIO Total 1,952,819.99 004965 FIRST VEHICLE SERVIC Total 1,723,635.88 009580 PETROLEUM TRADERS Total 1,583,193.04 000391 SCE&G COMPANY Total 1,397,240.49 008956 NEWTON BUILDERS Total 1,189,505.00 005687 SCDOT Total 1,133,904.00 005133 REPUBLIC WASTE SRV O Total 1,029,027.95 004400 MOTOROLA INC Total 1,021,502.87 001546 TOWN OF BLUFFTON Total 1,000,000.00 003976 STATE BUDGET AND CO Total 925,502.05 009424 CENTURYLINK Total 855,930.15 000210 HARGRAY TELEPHONE Total 818,942.33 007283 TERRY R. LEE CONTRAC Total 699,538.00 004575 JOHNSON CONTROLS INC Total 640,746.38 002510 CAROLINA CLEANING Total 620,374.12 007859 COLUMBIA FREIGHTLINE Total 612,501.00 005570 VIC BAILEY FORD Total 597,578.00 006961 SOUTHERN HEALTH PART Total 593,926.22 000547 HOWELL GIBSON & HUGH Total 593,215.04 004936 BANK OF AMERICA Total 592,166.02 007757 BURNS AUTOMOTIVE FOR Total 542,352.54 080272 SOLICITOR'S OFFICE Total 524,607.47 000982 WILBUR SMITH & ASSOC Total 523,407.04 009807 MANATRON, INC. Total 521,365.17 009182 FLORENCE AND HUTCHES Total 507,999.46 000635 PALMETTO ELECTRIC CO Total 478,854.61 009138 STANDARD INS CO Total 466,634.61 007307 HEWLETT PACKARD Total 445,344.96 002405 SOUTHERN WASTE SERVI Total 438,023.21 009149 UNITED CONCORDIA Total 429,394.81 005122 ABL MANAGEMENT INC. -

2017 ANNUAL CONFERENCE May 20 – 25, 2017 Crowne Plaza Atlanta Perimeter Atlanta, Georgia
Patent Information Users Group, Inc. The Complete 21st Century Patent Searcher — Addressing Our Skills Gaps 2017 ANNUAL CONFERENCE May 20 – 25, 2017 Crowne Plaza Atlanta Perimeter Atlanta, Georgia Meeting Book prepared by BizInt Solutions Inc., a proud sponsor of the PIUG 2017 Annual Conference 2017 ANNUAL CONFERENCE An International Conference for Patent Information Professionals May 20 – 25, 2017 Crowne Plaza Atlanta Perimeter Atlanta, Georgia Saturday, May 20, 2017 8:45 am 12:30 pm “Making It to the Premiership” – A Review of the Rising Stars in the IP Information Space (Stephen Adams) – FEE Maplewood 1:00 pm 4:00 pm The EPO Patent Information Products Road Show - 2017 (EPO) – Free Maplewood Sunday, May 21, 2017 8:00 am 9:00 am Global Patent Dossier (USPTO) – Free Maplewood 9:00 am 10:00 am Creating IP Reports with BizInt Smart Charts for Patents: Tips & Tricks (BizInt) – Free Dunwoody A 9:00 am 10:00 am A Revolution in Innovation: New Data and Tools for Improved Patent Analysis (Clarivate Analytics) – Free Dunwoody B 10:00 am 11:30 am Bridging the Gap between Searchers and Internal Clients with Evalueserve’s IP and R&D Dashboard (Evalueserve) Maplewood 11:30 am 12:30 pm Introducing PatSeer Pro–Serious analysis tools with great looking, flexible and interactive visualizations! (Gridlogics) Dunwoody C 11:30 am 1:30 pm The Road Ahead – Questel’s Latest Innovations, Partnerships, and Data Curating Techniques (Questel) – Free Oakwood 1:00 pm 2:00 pm Complexities in Sequence Searching (GQ Life Sciences) – Free Dunwoody A 1:00 pm 2:00 pm Broaden Your Patent Search Horizons With The Latest Solutions From Minesoft (Minesoft) – Free Dunwoody B 2:00 pm 3:30 pm Tech Mining Patents (Search Technology) – Free Maplewood 4:00 pm 5:30 pm PIUG Business Meeting (All conference attendees) Ravinia ABC 6:30 pm 7:30 pm First-Time Attendee Welcome & Orientation Reception (First Time PIUG Conference attendees) Camellia 7:30 pm 9:30 pm Opening Reception in Exhibit Hall – exhibits open (All conference attendees). -
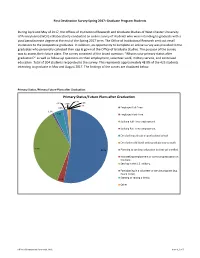
Primary Status/Future Plans After Graduation 0.0% 2.0% 0.5% 0.0% 1.0% 0.0% 0.0% Employed Full-Time
First Destination Survey Spring 2017: Graduate Program Students During April and May of 2017, the Offices of Institutional Research and Graduate Studies of West Chester University of Pennsylvania (WCU) collaboratively conducted an online survey of students who were intending to graduate with a post baccalaureate degree at the end of the Spring 2017 term. The Office of Institutional Research sent out email invitations to the prospective graduates. In addition, an opportunity to complete an online survey was provided to the graduates who personally collected their cap & gown at the Office of Graduate Studies. The purpose of the survey was to assess their future plans. The survey consisted of the broad question: “What is your primary status after graduation?” as well as follow-up questions on their employment, volunteer work, military service, and continued education. Total of 204 students responded to the survey. This represents approximately 48.0% of the 425 students intending to graduate in May and August 2017. The findings of the survey are displayed below. Primary Status /Primary Future Plans after Graduation: Primary Status/Future Plans after Graduation 0.0% 2.0% 0.5% 0.0% 1.0% 0.0% 0.0% Employed Full-Time. 1.0% 4.0% Employed Part-Time. Seeking Full-Time employment. Seeking Part-Time employment. Enrolled in graduate or professional school. Enrolled in additional undergraduate course work. 35.6% 53.0% Planning to continue education but not yet enrolled. Not seeking employment or continuing education at this time. Serving in the U.S. military. Participating in a volunteer or service program (e.g. -

2018 Annual Report
Annual Report January 2019 - Reporting on Fiscal Year 2018 As required by SC Code Section 57-3-760, to include 2017 Act No. 40, Section 57-1-380. Annual Report • • • Our Mission Annual Report • • • January 2019 - Reporting on Fiscal Year 2018 “SCDOT shall have as its functions and purposes the The South Carolina Department of Transportation (SCDOT) is one systematic planning, of the five largest state agencies in South Carolina and has a staff construction, maintenance, of approximately 4,500 men and women who work in all of the and operation of the state state’s 46 counties, with the central headquarters located in highway system and the Columbia. The agency’s purposes include planning, construction, development of a statewide maintenance, and operation of the state highway system, and the intermodal and freight development of a statewide intermodal and freight program. system … the goal of the A State Transportation Commission is the policy making body for Department is to provide SCDOT. It consists of nine-members – one member from each adequate, safe, and efficient Congressional District and two at-large members. The transportation services for Commission appoints the Secretary of Transportation who carries the movement of people and out the policies of the Commission, the daily operations of the goods.” agency and provides direction to staff. The Secretary of SCDOT recognizes that the statewide network of all SCDOT divisions, departments, and units in offices at (SC Code Section 57-1-30) Headquarters and throughout the Districts are one team – One SCDOT. In retrospect of the extraordinary events of the past year, the SCDOT workforce not only serves to accomplish the mission and achieve the vision, it has also embodied the following SCDOT values: Team Excellence Accountability Make a Difference 1 Annual Report • • • SECTION I- AGENCY ACCOMPLISHMENTS During the 2017-2018 fiscal year, SCDOT accomplished many milestones in “Year One” of the Ten Year Plan and earned major achievements and awards. -

Collaborate to Innovate
DIA 2012 Collaborate to Innovate FINAL PROGRAM 48th Annual Meeting June 24-28 | Philadelphia, PA Pennsylvania Convention Center www.diahome.org/DIA2012 Craig H. Lipset Head of Clinical Innovation, Worldwide Research & Development, Michael A. Nutter Pfizer Inc Mayor of Philadelphia Dear Colleagues, Welcome to Philadelphia and to DIA’s 48th Annual Meeting. Thank you for attending the DIA 2012 48th Annual Meeting. I am thrilled that the DIA 2012 48th Annual The theme of this year’s Annual Meeting is Collaborate to Innovate. What Meeting Collaborate to Innovate is being does this mean? It means that over the next few days you will attend and held right here in the Birthplace of American participate in tutorials, sessions, workshops, symposia, and panel discus- Medicine. Philadelphia is the perfect setting sions to address the collaboration required among diverse partners to for your gathering. It is home to some of the bring innovative new medical products to patients. world’s most renowned institutions of higher learning, where the best and the brightest To me, that is what the DIA Annual Meeting is all about—putting new minds are leading the quest for new medical ideas into practice, producing rich content, offering robust networking discoveries and innovations. opportunities, and providing access to the world’s most innovative tech- nology partners—so we can continue to make an important impact in the Philadelphia is fortunate to have DIA’s delivery of new therapies to patients. global headquarters located just a few miles outside Center City—making it a part of the "Innovation" is a very popular buzzword, but it’s more than just good fabric of our great city. -

DCTS Meeting Minutes
MINUTES DELAWARE COUNTY INTERMEDIATE UNIT BOARD MEETING Acting as Agent for DELAWARE COUNTY AREA VOCATIONAL-TECHNICAL SCHOOL BOARD October 7, 2020 A meeting of the Technical Schools Board was called to order at 7:44 p.m. by President Edward Cardow. The meeting was held in hybrid mode due to the COVID-19 quarantine. Some board members attended in person at 200 Yale Avenue, Morton, PA 19070, and some members attended virtually via Zoom. Board Members Present Nonmembers Present Mr. Christopher Bryan (IP) Dr. Maria Edelberg, Executive Director (IP) Mr. Edward J. Cardow (IP) Mr. Thomas Brown, Treasurer (IP) Ms. Hillary Fletcher (V) Dr. Shellie Feola, Secretary (IP) Ms. Amy Goldman (V) Mr. Michael Puppio, Esq. (V) Mr. Edward Harris (IP) Ms. Joi Hopkins (V) Ms. Tracy Karwoski (IP) Mr. Lawrence Kutys (IP) Ms. Susan Mingey (IP) Ms. Rachel Mitchell (V) Ms. Sheree Monroe (IP) Ms. Margaret Rovinski (IP) Absent were: Ms. Barbara Harvey Mr. Anthony Johnson Ms. M. Colleen Powell (Note: IP=in-person attendance; V=virtual attendance) Dr. Edelberg sat as the executive officer of the Board. A quorum was present. Ms. Monroe moved and Mr. Harris seconded the motion to accept the resignation of Dr. Michele Downie, Board representative for the Wallingford-Swarthmore School District, effective August 26, 2020. Voting: 11-0, Motion Approved Ms. Karwoski moved and Ms. Rovinski seconded the motion to appoint Mr. Lawrence Kutys, Wallingford-Swarthmore School District, to fulfill the term August 27, 2020 through June 30, 2021, vacated by Dr. Michele Downie. Voting: 11-0, Motion Approved DCAVTS Minutes October 7, 2020 Minutes of September 2, 2020 Meeting The minutes of the September 2, 2020 meeting were approved, without reading, following a motion made by Ms. -

2013 Annual Conference an International Conference for Patent Information Professionals Saturday, April 27 – Thursday May 2, 2013
PATENT INFORMATION USERS GROup, INC. Celebrating Our Past 25 Years...Preparing for Our Future Best Practices for Keeping Up with the Rapidly Changing Patent Landscape 2013 Annual Conference An International Conference for Patent Information Professionals Saturday, April 27 – Thursday May 2, 2013 Hilton Alexandria Mark Center • 5000 Seminary Road • Alexandria, VA Meeting Book prepared by BizInt Solutions Inc., a proud sponsor of the 2013 PIUG Annual Conference April 27 - May 2, 2013 2013 Annual Conference Hilton Alexandria Mark Center Alexandria, VA Saturday, April 27th, 2013 8:00 am 12:00 noon The Importance of Presenting Useable Search Output to the Client (Stephen Adams) - FEE Magnolia B 1:00 pm 5:00 pm Fundamentals of Current Patent Law (Edlyn Simmon) – FEE Magnolia B 8:00 am 4:00 pm Patinformatics: The Next Generation (Tony Trippe) – FEE Magnolia A 1:30 pm 4:30 pm Personal Branding: Communicating the Authentic You – Free Magnolia C Sunday, April 28th, 2013 8:30 am 10:00 am Creating Patent Reports and Visualizations with BizInt Smart Charts (Bizint Solutions) – Free Terrrace East 10:00 am 12:00 pm Soaring into Orbit.com with Perfected Search and Analysis Tools (Questel) – Free Arbors 10:30 am 11:30 am Making IP Intelligence Easy and Intuitive (Relecura) – Free Terrace West 11:00 am 12:00 pm Exclusive Sneak Peek: ProQuest Dialog™ Patents (ProQuest Dialog) – Free Terrace East 12:00 pm 1:00 pm Product Updates and Enhancements (Minesoft) – Free Terrace West 12:30 pm 1:30 pm Searching in DWPI Chemistry Resource (DCR) on STN (STN) – Free Terrace East 2:00 pm 3:30 pm Thomson Innovation: Enhanced Sources & Insight 2013 (Thomson Reuters) – Free Arbors 4:00 pm 5:30 pm PIUG Business Meeting (All PIUG members are encouraged to attend) Plaza Ballroom East 6:30 pm 7:30 pm First-Time Attendees Welcome & Orientation. -
FULL NAME Contact Company ID Email 3M Company Suzanne Danielson CC0013 [email protected] 3M Unitek Corporation Vincent Martinez CC0361 [email protected] A-Dec, Inc
FULL_NAME Contact Company_ID Email 3M Company Suzanne Danielson CC0013 [email protected] 3M Unitek Corporation Vincent Martinez CC0361 [email protected] A-dec, Inc. Anh Vo CC0721 [email protected] ARIAD Pharmaceuticals Chad A. Morin CC0645 [email protected] ATS Medical Astrid Berthe CC0414 [email protected] AbbVie, Inc. Lynne Inman CC0690 [email protected] Abbott Laboratories Joanne Lee CC0017 [email protected] Abiomed Inc. Stephen McEvoy CC0038 [email protected] Abraxis Bioscience Inc. Charles Kim CC0039 [email protected] Acadia Pharmaceuticals, Inc. Ryan Brown CC0830 [email protected] AccessClosure, Inc. John Buckley CC0541 [email protected] Acclarent Inc. Susan Clarke CC0040 [email protected] Accuray Incorporated Alaleh Nouri CC0692 [email protected] Acorda Therapeutics, Inc. Naresha Moore CC0041 [email protected] Actavis, PLC Alicia Riegle CC0376 [email protected] Actelion Pharmaceuticals U.S., Inc. Nimit Gajera CC0042 [email protected] Actient Pharmaceuticals LLC Steve Griffin CC0617 steve.griffin@actientpharma. Acumed Llc Tony Damas CC0026 [email protected] Adolor Corporation Elizabeth Jobes CC0043 [email protected] Advanced BioHealing, Inc. Bill Benvenuto CC0413 [email protected] Advanced Bionics Darci Teobaldi CC0044 darci.teobaldi@advancedbioni Advanced Orthopaedic Solutions Inc. Anna Hwang CC0655 [email protected] Advandx Inc. Emad Lababidi CC0023 [email protected] Aegerion Pharmaceuticals, Inc. Deidre Arnold CC0647 [email protected] Aesculap Implant Systems, Inc. Karen Smickley CC0034 karen.smickley@aesculap. Aesculap Inc. Karen Smickley CC0033 karen.smickley@aesculap. Afaxys, Inc. Nancy Duncan CC0657 [email protected] Agency For Medical Innovations Andrew Bendheim CC0372 [email protected] Agency for Medical Innovations Inc. -

Company Country - Canada F
Company Country - Canada F. Hoffmann-La Roche AG Switzerland Retail Business Services, an Ahold Delhaize company United States #TheKrewe United States . United Kingdom ... United States @2toLead Canada @GrumpyTech United States @PatrikWennberg Sweden @peterbb Denmark @rob_rich United States @WinnCompanies - Boston, MA United States [s.i.g.] mbH Germany _ United States ______________ United States ______________________________________ United States 10th magnitude United States 1105 Media United States 1105 Media, Inc. United States 1105 Redmond Media Group United States 14 West United States 14 West Global Technologies United States 14West Global Technology United States 1-800 CONTACTS United States 1800 CONTACTS INC United States 1-800Contacts United States 1ClickFactory Lithuania 1E United States 1E Inc United States 1E, Inc. United States 1plex United Kingdom 1st Source Bank United States 1stQuad Solutions Switzerland 2Pint Software Sweden 2toLead Canada 356labs Bulgaria 365View Canada 38 South Pty Ltd Australia 3bits Consulting AB Sweden 3CX United States 3D Exhibits United States 3D Systems United States 3E Company United States 3F Denmark 3FT United States 3M United States 3M Company United States 3M Corp United States 3M HIS United States 3Sharp United States 3Sharp LLC United States 451 Research Canada 451 Research United Kingdom 4ward srl Italy 4ward USA United States 4ward365 France 4ward365 srl Italy 5 Point Enterprises / New Horizons Computer Learning Centers United States 5nine Inc United States 5nine Software United States 5nine, Inc. United States 6th Street Consulting United States 7-Eleven, Inc. United States 84.51 United States 8451 United States 8x8 United States 9 Dots Consulting Group Malaysia 99 Cents Only Stores LLC United States 9DW Inc. -

Case 1:20-Cv-00706-DLC Document 229 Filed 08/18/20 Page 1 of 43
Case 1:20-cv-00706-DLC Document 229 Filed 08/18/20 Page 1 of 43 UNITED STATES DISTRICT COURT SOUTHERN DISTRICT OF NEW YORK -------------------------------------- X : FEDERAL TRADE COMMISSION, STATE OF NEW : YORK, STATE OF CALIFORNIA, STATE OF : OHIO, COMMONWEALTH OF PENNSYLVANIA, : 20cv706 (DLC) STATE OF ILLINOIS, STATE OF NORTH : CAROLINA, and COMMONWEALTH OF : OPINION AND ORDER VIRGINIA, : : Plaintiffs, : : -v- : : VYERA PHARMACEUTICALS, LLC, AND : PHOENIXUS AG, MARTIN SHKRELI, : individually, as an owner and former : director of Phoenixus AG and a former : executive of Vyera Pharmaceuticals, : LLC, and KEVIN MULLEADY, individually, : as an owner and former director of : Phoenixus AG and a former executive of : Vyera Pharmaceuticals, LLC, : : Defendants. : : -------------------------------------- X APPEARANCES For plaintiff Federal Trade Commission: Markus H. Meier Bradley S. Albert Armine Black Daniel W. Butrymowicz D. Patrick Huyett Neal J. Perlman J. Maren Schmidt James H. Weingarten Federal Trade Commission 600 Pennsylvania Avenue, NW Washington, DC 20580 (202) 326-3748 For plaintiff State of New York: Letitia James Christopher D’Angelo Case 1:20-cv-00706-DLC Document 229 Filed 08/18/20 Page 2 of 43 Elinor R. Hoffman Saami Zain Amy McFarlane Jeremy Kasha Bryan Bloom Office of the New York Attorney General Antitrust Bureau 28 Liberty Street, 20th Floor New York, NY 10005 (212) 416-8262 For plaintiff State of California: Michael D. Battaglia Office of the Attorney General of California 455 Golden Gate Avenue, Suite 11000 San Francisco, CA 94102 (415) 510-3769 For plaintiff State of Ohio: David Yost Beth Finnerty Elizebeth M. Maag Office of the Ohio Attorney General 150 E. Gay Street, 22nd Floor Columbus, OH 43215 (614) 466-4328 For plaintiff Commonwealth of Pennsylvania: Josh Shapiro Tracy W. -

DIA 2016 Final Program
FINAL PROGRAM HELPING DELIVER LIFE CHANGING THERAPIES “We need more clinical trials and we need to keep innovating in clinical research.” JUNE 26–30 | PHILADELPHIA, PA The Pennsylvania Convention Center Teresa Triple Negative Breast Cancer DIAglobal.org/DIA2016 Survivor and Executive Director, Project Management, PPD A GATHERING OF GLOBAL PERSPECTIVES OF GLOBAL A GATHERING “I have diabetes and I’m happy to know that people are working to find a cure.” Luke 12 Year Old Living with Type I Diabetes At PPD, what we do impacts our clients, our employees and people around the world. We see the benefits of clinical trials firsthand and are committed to building strong partnerships A GATHERING OF GLOBAL PERSPECTIVES with our clients. Because to us, clinical research isn’t just business — it’s personal. Be part of the change. Visit PPD at Booth No. 701 and see how you can help change lives. www.ppdi.com | #TogetherWithPPD © 2016 Pharmaceutical Product Development, LLC. All rights reserved. Every Touchpoint, Every Way That Matters. Commercial and Clinical Teams Field • Multi-channel • Contact Center Support Services/Individual Solutions Recruiting • Training • Sales and Clinical Operations Advanced Analytics • Compliance Consulting Consistently delivering extraordinary results for your brand from Pre-Launch through LOE TouchpointSolutions.com • 1-866-616-4777 • impact@ TouchpointSolutions.com Final Program Follow #DIA2016 for real-time updates Table of Contents Schedule At-A-Glance ........................................ 2 Program Highlights ...............................................3