Cation Distribution in Partially Ordered Columbite from the Kings Mountain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Wodginite Group. Iii. Classification and New Species
633 Canadian Mineralogist Vol. 30, pp. 633-638(1992) THE WODGINITE GROUP. III. CLASSIFICATION AND NEW SPECIES T. SCOTT ERCITI, PETRIERNY eNp FRANK C, HAWTHORNE Deportmentof GeologicalSciences, University of Manitoba, Winnipeg,Manitoba R3T 2N2 ABSTRACT The minerals constituting the wodginite group are isostructural oxides, with the generalformula ABC2Oy (Z = 4);A = Mn2*, Fd*, Li, E B : Sn4*, Ti, Fe3*, Ta;C : Ta, Nb. A classificationsystembased onthe chemistry of the .,4-and B-cationsites has been devised; it useschemical modifiers in conjunctionwith the root name"wodginite". Wodginite itself has the generalformula MnSnTa2Os;two new membersof the wodginite group are charactedzed here:-ferrowodginite, F/+SnTa2Os and titanowoiginite, MnTiTa2Os. Ferrowodginite occurs as 0.01- to- 0.2-mm inclusionsin cassiteritefrom a granitic pegmatitenear Sukula, southwestemFinland. It is dark brown to black' has a dark brown streak, vitreous luster, is optically anisotropic with all n>2, and is nonfluorescent.The calculated density is 7.02 g/cm3; it is brittle, H 5%, without cleavage;it has an irregular fracture. The averagechemical composition(wt. Vo)is: MnO 2.8, FeO 9.3, Fe2O31.9, TiO2 4.3, SnO210.1, Nb2O5 14.8, Ta2O5 56.3, total 99.4.The strongestfivelines in the X-ray powderpauein-are:4.16('50),'2.g7(Aq,2.55(30i, 2.493(40\ {nd 1.a55(30)A. fne unit-cell parametersare o 9.415(7),b 11.442(6),c 5.103(4)A, B 90.8(l)', V 549.'7(6)Ar, spacegroup A/c. Titanowodginiteoccurs as euhedral,diamond-shaped crystals up to I cm acrossat the Tanco pegmatite,Bernic Lake, Manitoba. -

The Crystal Ghemistry of Complex Niobium and Tantalum Oxides Ll. Composition and Structure of Wodginite
American Mineralogist, Volume 59, pages 1040-1044, 1974 TheCrystal Ghemistry of ComplexNiobium and Tantalum Oxides ll. Compositionand Structure of Wodginite J. Gnermu, axo M. R. TnonNsnn Diaision of Mineralogy, CSIRO, Floreat Park, Iilestern Australia, 6014 Abstract The structure of wodginite (non-centrosymmetric monoclinic space group Cc; ideal formula, MnXtX[lao,, where site Xg contains 5-valent ions (and all the niobium) and the average valency of cations in site X, is 4) was determined by film methods to be an ordered form of tho ixiolite structure. The a and D cell edges and angle B increase with manganesb content, whereas c remains constant for wodginites from five localities in Western Australia. Composition and Lattice Parameters from Wodgina, hand picked from a homogeneous The charasteristicsof the mineral wodginite were region of a polished section.Electron probe analysis clearly describedby Nickel, Rowland, and McAdam and powderX-ray diffraction (Hiigg Guinier focussing (1963) for samplesof the mineral from Wodgina, camera) gave the cell dimensibnsand composition Australia, and Bernic Lake, Manitoba. The s,truc- usedin the calculationsshown in Table l. Data were ture was not fully defined, and the efiect of com- collectedphotographically along two axes([010] and positionalvariations on the st,ructurecould not there- Ul2l) using a Nonius Integrating Weissenberg fore be discussed. camera, equiinclination geometry,and CuKa radia- Table 1 shows the compositions and lattice tion. After the applicationof Lorenz and polarization parametersof wodginitesfrom five localitiesin West- corrections, these two sets of data were inter-cor- ern Australia and the averageparameters quoted by related, and symmetrically similar reflections were Grice, eerny and Ferguson (1972) fbr the wod- averagedto give 555 independentobservations. -

And Ta-Bearing Oxide Minerals in the Greer Lake Pegmatitic Granite and Its Pegmatiteaureole, Southeasternmanitoba
American Mineralogist, Volume 71, pages 501-517, 1986 Fractionation trends of the Nb- and Ta-bearing oxide minerals in the Greer Lake pegmatitic granite and its pegmatiteaureole, southeasternManitoba Pnrn ennmi, Bnucn E. Golo,r FuNx C. H,lwruronNr, RoN CrHplr,lN Department of Earth Sciences,University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada Arsrucr The Greer Lake pegmatitic granite and related exterior rare-element pegmatites of the beryl-columbite type intrude metabasalt and tonalite gneiss in the Archean English River Subprovince of southeasternManitoba. Columbite-tantalite is the predominant Nb- and Ta-bearingmineral, associatedwith subordinateixiolite, microlite, niobian-tantalian rutile, and rare tantalian cassiterite, wodginite, and ilmenite. In coexisting mineral pairs, Tal(Ta + Nb) of microlite exceedsthat of columbite-tantalite, ixiolite, rutile, and cassiterite;in cassiteriteand rutile, Tal(Ta + Nb) is higher and Mn/(Mn * Fe) lower than in columbite- tantalite and ixiolite. In Li-, Rb-, Cs-, and F-poor environments, limited Mn enrichment accompaniesthe fractionation of Ta, which culminates in ixiolite and subordinate microlite. In Li-, Rb-, Cs-, and F-rich parageneses,extensive Mn enrichment precedesthe main Ta fractionation, which subsequentlygenerates near-end-member manganotantalite, wodginite, and mi- crolite. The Greer Lake and other fractionation trends indicate that a late-stageF-rich environment promotes extreme Fe/Mn fractionation prior to the main stage of Nb-Ta separation. The abundancesof Sn, Ti, and Sc are not related to fluorine or rare-alkali enrichment, but increasefrom pegmatitic granite to pegnatites. The relative accumulation of Ti and Sc in the pegmatite aureole seemsto be due to internal fractionation rather than assimilation. Columbite-tantalite in the pegmatitic granite shows an intermediate to near-ordered structural state, but is highly disordered in the pegmatite veins. -

Niobian Rutile from the Mcguire Granitic Pegmatite, Park County, Colorado: Solid Solution, Exsolution, and Oxidation
American Mineralogist, Volume 84, pages 754–763, 1999 Niobian rutile from the McGuire granitic pegmatite, Park County, Colorado: Solid solution, exsolution, and oxidation PETR Cˇ ERNY´ ,1,* RON CHAPMAN,1 WILLIAM B. SIMMONS,2 AND LEONARD E. CHACKOWSKY1 1 Department of Geological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada 2 Department of Geology and Geophysics, University of New Orleans, New Orleans, Louisiana 70148-2850, U.S.A. ABSTRACT Coarse crystals of niobian rutile occur in the hydrothermally altered core-margin zone of the McGuire granitic pegmatite, Park County, Colorado, associated with potassium feldspar, quartz, bi- otite, ilmenite, and monazite-(Ce). Primary homogeneous niobian rutile, with Fe3+ @ Fe2+ and a small excess of (Fe,Mn) over the amount required to compensate the incorporation of (Nb,Ta,W), under- went three stages of exsolution. Primary homogeneous niobian rutile exsolved a fine trellis-like pat- tern of minor lamellar Nb-bearing pseudorutile I. Most of this phase was broken down to pseudomor- phs consisting of microgranular Nb-rich pseudorutile II imbedded in niobian-ferrian “ferropseudobrookite.” Continued exsolution in niobian rutile and reconstitution of the early exsolution products generated (Fe,Nb)-depleted, microgranular niobian rutile, titanian ferrocolumbite, and mi- nor ilmenite. These three phases did not attain chemical equilibrium but may represent a stable phase assemblage. All these processes seem to have maintained charge balance, suggesting a closed sys- tem. Subsequent to the three stages of exsolution, extensive oxidation converted the mineral assem- blages to anatase + hematite + titanian-tungstenian ixiolite; primary ilmenite was oxidized into an anatase + hematite intergrowth. In both cases, the hematite component was almost completely leached out, leaving highly porous aggregates of the other phases. -

First U.S. Occurrence of Wodginite from Powhatan County, Virginia
American Mineralogist, Volume 69, pages 807-809, 1984 First U.S. occurrenceof wodginitefrom PowhatanCounty, Virginia MrcHeel A. WIse eNo Pnrn er,nNf Department of Earth Sciences, University of Manitoba Winnipeg, Manitoba R3T 2N2 Canada Abstract Wodginite occurs as black subhedral grains in the Be, Nb-Ta, Sn bearing Herbb #2 pegmatite in Powhatan County, Virginia. The wodginite is associatedwith amazonite and topaz in the intermediate zone of the pegmatite. Other Nb-Ta-bearing minerals found associated with wodginite are cassiterite, in part as inclusions in wodginite, and par- tially ordered man6ianocolumbite.The Herbb wodginite is compositionally similar to previ- ously studied wodginitesand has an averageformula (Mnz.aoFe?.1sb+.or(Snz.oqTirrcTaost Fd.14)x.8e(Ta7.ozNbos8)>8.0oOrz.The average unit cell dimensions ate a 9.471(2), b tt.43t(2),c 5.108(l)A, p90"47'(t),v 553.1(l)43. Introduction ite), quartz and muscovite are the dominant minerals present. Beryl, topaz, columbite-tantalite,wodginite, A black mineral, at first believed to be columbite- cassiterite,and minor spessartinealso occur in the peg- tantalite, was collected by the first author from the Herbb matite, mainly in the clayey decomposition products. #2 pegmatite in Powhatan County, central Virginia. Wodginite is found scatteredthroughout the kaolinized Griffitts et al. (1953) noted "small lustrous tabular to feldsparof the intermediatezones. Veinlets of wodginite equant crystals of columbite-tantalite"in albitized por- also occur near perthitic amazonitebodies and to a lesser tions of this pegmatite,a speciesalso quotedby Jahnset eitent are also associatedwith topaz. al. (1952).Recent examination of Nb,Ta-bearingminerals from this locality has led to the identification of the rare Physical properties Mn,Sn,Ta-oxidemineral, wodginite. -

The Crystal Chemistry of the Tapiolite Series
631 The Canadian M ineralo g is t Vol. 34, pp.631,-647(1,996) THE CRYSTALCHEMISTRY OFTHE TAPIOLITE SERIES MICHAELA. WISE Departnunt of Mincral Sciences,Snr.ithsonian hstitution" Washington,D,C, 20560, U.SA. PETR dERNf Depamnentof GeologicalSciences, Unitersity of Maninba. Winnipeg,Manitoba R3T2N2 ABsrRAc"r Ferotapiolite-manganotapiolite-groupminslzls [@e,Mn)Ta2O6]axe formed as :rccessoryphases in rare-elementgranitic pegmatitesexhibiting moderateto high degreesof fractionation.Despite their relative paucity, 94 setsof unit-cell parameters and 194 chemical compositionswere compiled to characterizethe crystal chemistry of the tapiolite series.X-ray-diffraction studiesindicate that the degreeof orderand compositional variations stongly influenceunit-cell dimensionsof tapiolite.Natural tapiolite showswide rangesof structuralstate. Crystallization of disorderedphases at low lemperaturesis suggested;however, the available data are not unambiguous.Tapiolite chemistry is typically uniform (Fe > Mn, Ta > Nb), and compositional variations are the result of limited, but effective homovalentard heterovalentsubstitutions: (1) Mn2+Fe2+-r;(2) NbsiTas+-l; (3) Fek(ti,Sn)4rFe2+_r(IaM)5*-r and (4) @,sn)4,1Fe,Mn;{,6M,Ta)5*_2. ln association witl otler M,Ta,Sn-bearing minerals,tapiolite shows a distinct preferencefor Fd+ and Ta over Mn and Nb. Emicbment in Nb, Ti and Sn appearsto be commonin tapiolite from moderatelyfractionated pegmatites, whereas extreme Mn enrichmentis typical of highly fractionated pegmatitesof the petalite subtypeor metasomaticunits. Keywords:tapiolite series,crystal chemistry,order - disorder,tantalum" granitic pegmatite. SoM:uaRs Irs mindraux de la s6de ferrotapiolite - manganotapiolitetGe"IUn)TqO6l se pr6sententsous forme d'accessoiresdes pegmatitesgranitiques d 6l6mentsrares faisantpreuve d'un degr6de fractionnementintermddiaire tr 61ev6.Malgrd leur raret6, les r6sultatsde 94 affinementsde la maille 6l6mentaireet de 194 analyseschimiques sont disponibles,et serventi caract6riser la cristallochimie des min6raux de cette s6rie. -

Crystallization Processes and Solubility of Columbite-(Mn), Tantalite-(Mn), Microlite, Pyrochlore, Wodginite and Titanowodginite in Highly Fluxed Haplogranitic Melts
Western University Scholarship@Western Scholarship@Western Electronic Thesis and Dissertation Repository 3-12-2018 10:30 AM Crystallization processes and solubility of columbite-(Mn), tantalite-(Mn), microlite, pyrochlore, wodginite and titanowodginite in highly fluxed haplogranitic melts Alysha G. McNeil The University of Western Ontario Supervisor Linnen, Robert L. The University of Western Ontario Co-Supervisor Flemming, Roberta L. The University of Western Ontario Graduate Program in Geology A thesis submitted in partial fulfillment of the equirr ements for the degree in Doctor of Philosophy © Alysha G. McNeil 2018 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Earth Sciences Commons Recommended Citation McNeil, Alysha G., "Crystallization processes and solubility of columbite-(Mn), tantalite-(Mn), microlite, pyrochlore, wodginite and titanowodginite in highly fluxed haplogranitic melts" (2018). Electronic Thesis and Dissertation Repository. 5261. https://ir.lib.uwo.ca/etd/5261 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract Niobium and tantalum are critical metals that are necessary for many modern technologies such as smartphones, computers, cars, etc. Ore minerals of niobium and tantalum are typically associated with pegmatites and include columbite, tantalite, wodginite, titanowodginite, microlite and pyrochlore. Solubility and crystallization mechanisms of columbite-(Mn) and tantalite-(Mn) have been extensively studied in haplogranitic melts, with little research into other ore minerals. A new method of synthesis has been developed enabling synthesis of columbite-(Mn), tantalite-(Mn), hafnon, zircon, and titanowodginite for use in experiments at temperatures ≤ 850 °C and 200 MPa, conditions attainable by cold seal pressure vessels. -

Tantalum Mineralogy, Rare-Element Granitic Pegmatites, Separation Lake
Ontario Geological Survey Open File Report 6022 Tantalum Mineralogy of Rare-Element Granitic Pegmatites from the Separation Lake Area, Northwestern Ontario 2000 ONTARIO GEOLOGICAL SURVEY Open File Report 6022 Tantalum Mineralogy of Rare-Element Granitic Pegmatites from the Separation Lake Area, Northwestern Ontario by A.G. Tindle and F.W. Breaks 2000 Parts of this publication may be quoted if credit is given. It is recommended that reference to this publication be made in the following form: Tindle, A.G. and Breaks, F.W. 2000. Tantalum mineralogy of rare-element granitic pegmatites from the Separation Lake area, northwestern Ontario; Ontario Geological Survey, Open File Report 6022, 378p. e Queen’s Printer for Ontario, 2000 e Queen’s Printer for Ontario, 2000. Open File Reports of the Ontario Geological Survey are available for viewing at the Mines Library in Sudbury, at the Mines and Minerals Information Centre in Toronto, and at the regional Mines and Minerals office whose district includes the area covered by the report (see below). Copies can be purchased at Publication Sales and the office whose district includes the area covered by the report. Al- though a particular report may not be in stock at locations other than the Publication Sales office in Sudbury, they can generally be obtained within 3 working days. All telephone, fax, mail and e-mail orders should be directed to the Publica- tion Sales office in Sudbury. Use of VISA or MasterCard ensures the fastest possible service. Cheques or money orders should be made payable to the Minister of Finance. Mines and Minerals Information Centre (MMIC) Tel: (416) 314-3800 Macdonald Block, Room M2-17 1-800-665-4480(toll free inside Ontario) 900 Bay St. -
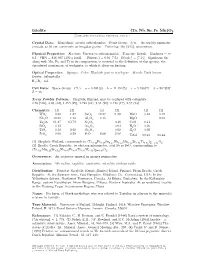
Ixiolite (Ta, Nb, Sn, Fe, Mn)O2 C 2001-2005 Mineral Data Publishing, Version 1
Ixiolite (Ta, Nb, Sn, Fe, Mn)O2 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic, pseudo-orthorhombic. Point Group: 2/m. As crudely prismatic crystals, to 10 cm; commonly as irregular grains. Twinning: On {013}, uncommon. Physical Properties: Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 6– 6.5 VHN = 836–967 (100 g load). D(meas.) = 6.94–7.23 D(calc.) = [7.34] Significant Sn, along with Mn, Fe, and Ti in its composition, is essential to the definition of this species, the disordered counterpart of wodginite, to which it alters on heating. Optical Properties: Opaque. Color: Blackish gray to steel-gray. Streak: Dark brown. Luster: Submetallic. R1–R2: n.d. Cell Data: Space Group: P 2/c. a = 9.481(3) b = 11.494(5) c = 5.158(2) β =90◦8(2)0 Z=16 X-ray Powder Pattern: Skogb¨ole,Finland; may be confused with columbite. 2.98 (100), 3.65 (32), 1.459 (29), 1.722 (24), 2.51 (20), 1.746 (17), 2.57 (13) Chemistry: (1) (2) (1) (2) (1) (2) WO3 0.30 1.87 SnO2 12.27 11.38 MnO 5.40 9.19 Nb2O5 10.50 6.12 Al2O3 0.16 MgO 0.01 Ta2O5 61.47 63.79 Sc2O3 0.16 CaO 0.11 + SiO2 0.12 As2O3 0.04 H2O 0.16 − TiO2 0.38 2.68 Sb2O3 0.02 H2O 0.08 ZrO2 0.60 0.20 FeO 8.08 2.98 Total 99.63 98.44 (1) Skogb¨ole,Finland; corresponds to (Ta0.44Fe0.18Sn0.13Nb0.12Mn0.12Zr0.01Ti0.01)Σ=1.01O2. -

Exsolution of Zirconian-Hafnian Wodginite from Manganoan-Tantalian Cassiterite, Annie Claim #3 Granitic Pegmatite, Southeastern Manitoba, Canada
685 The Canadian Mineralogist Vol. 38, pp. 685-694 (2000) EXSOLUTION OF ZIRCONIAN-HAFNIAN WODGINITE FROM MANGANOAN-TANTALIAN CASSITERITE, ANNIE CLAIM #3 GRANITIC PEGMATITE, SOUTHEASTERN MANITOBA, CANADA MORGAN MASAU, PETR CERNˇ Y´ § AND RON CHAPMAN Department of Geological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada ABSTRACT Primary (Mn,Fe,Ta,Nb)-bearing cassiterite with ≤0.21 wt.% ZrO2 and ≤0.05 wt.% HfO2 crystallized during the consolidation of the Archean Annie Claim #3 zoned, lepidolite-subtype granitic pegmatite, southeastern Manitoba, in amounts increasing from the outer to the inner pegmatite zones. Rare inclusions of tantalite and ferrotapiolite originated from transient local saturation in these phases. Sparse early cassiterite from the outer intermediate zone is rather rich in substituting elements and in part Fe- dominant, whereas the more abundant late cassiterite from the lepidolite core is strongly Mn-dominant and poor in the substituents. Exsolution in the primary cassiterite generated granular zirconian-hafnian ferrowodginite and wodginite enclosed in veinlets of substituent-depleted cassiterite. The Fe/Mn and Nb/Ta values of the wodginite phases mimic those of the primary cassiterite across the pegmatite zones, and Zr/Hf perceptibly decreases from the outer zones inward. Maximum contents of ZrO2 and HfO2 (5.98 and 1.59 wt.%, respectively; 0.31 Zr and 0.04 Hf apfu) are the highest so far encountered in wodginite-group minerals. These maxima characterize wodginite exsolved from early cassiterite; the levels drop off in wodginite exsolved in late cassiterite. Instead, hafnian zircon intergrown with abundant thorian coffinite becomes abundant in the lepidolite core, indicating a transition of Zr + Hf from octahedral sites in the oxide minerals to the much more common 8-fold coordination in the orthosilicate; this shift implies a transition from relatively alkaline to more acidic conditions. -

Primary Minerals of the Jáchymov Ore District
Journal of the Czech Geological Society 48/34(2003) 19 Primary minerals of the Jáchymov ore district Primární minerály jáchymovského rudního revíru (237 figs, 160 tabs) PETR ONDRU1 FRANTIEK VESELOVSKÝ1 ANANDA GABAOVÁ1 JAN HLOUEK2 VLADIMÍR REIN3 IVAN VAVØÍN1 ROMAN SKÁLA1 JIØÍ SEJKORA4 MILAN DRÁBEK1 1 Czech Geological Survey, Klárov 3, CZ-118 21 Prague 1 2 U Roháèových kasáren 24, CZ-100 00 Prague 10 3 Institute of Rock Structure and Mechanics, V Holeovièkách 41, CZ-182 09, Prague 8 4 National Museum, Václavské námìstí 68, CZ-115 79, Prague 1 One hundred and seventeen primary mineral species are described and/or referenced. Approximately seventy primary minerals were known from the district before the present study. All known reliable data on the individual minerals from Jáchymov are presented. New and more complete X-ray powder diffraction data for argentopyrite, sternbergite, and an unusual (Co,Fe)-rammelsbergite are presented. The follow- ing chapters describe some unknown minerals, erroneously quoted minerals and imperfectly identified minerals. The present work increases the number of all identified, described and/or referenced minerals in the Jáchymov ore district to 384. Key words: primary minerals, XRD, microprobe, unit-cell parameters, Jáchymov. History of mineralogical research of the Jáchymov Chemical analyses ore district Polished sections were first studied under the micro- A systematic study of Jáchymov minerals commenced scope for the identification of minerals and definition early after World War II, during the period of 19471950. of their relations. Suitable sections were selected for This work was aimed at supporting uranium exploitation. electron microprobe (EMP) study and analyses, and in- However, due to the general political situation and the teresting domains were marked. -

Mineralogy and Petrology of the Amazonite Pegmatite at Bakstevalåsen, Øvre Eiker
Master Thesis, Department of Geosciences Mineralogy and petrology of the amazonite pegmatite at Bakstevalåsen, øvre Eiker Øyvind Sunde Mineralogy and petrology of the amazonite pegmatite at Bakstevalåsen, øvre Eiker Øyvind Sunde Master Thesis in Geosciences Discipline: Geology Department of Geosciences Faculty of Mathematics and Natural Sciences University of Oslo July 2013 © Øyvind Sunde, 2013 Supervised by associate prof. Rune S. Selbekk and prof. Tom Andersen Cover picture: Hand specimen of the amazonite pegmatite at Bakstevalåsen measuring a 15 cm cross-section with amazonite matrix and abundant danalite. This work is published digitally through DUO – Digitale Utgivelser ved UiO http://www.duo.uio.no It is also catalogued in BIBSYS (http://www.bibsys.no/english) All rights reserved. No part of this publication may be reproduced or transmitted, in any form or by any means, without permission. Acknowledgements This thesis marks the end of a 5 –year period of time with relentless studies at the Department of Geosciences, University of Oslo. There are many people I have met during this 5-year ride who in various ways have contributed in shaping my interest for geology. I have never, ever, regretted my decision on setting sail onto this journey. You all know who you are and a huge thank you! My thesis would not have been possible without the help of several clever individuals, and I would like to aim a special appreciation to the following personnel: Rune Selbekk: first of all, thank you for letting me volunteer at the natural History Museum during my infant years of studying. It brought more geology into a curriculum diluted with meteorology and philosophy.