Tantalum Mineralogy, Rare-Element Granitic Pegmatites, Separation Lake
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solvent Extraction of Scandium from Pregnant Leach Solution of Nickel Laterite
SOLVENT EXTRACTION OF SCANDIUM FROM PREGNANT LEACH SOLUTION OF NICKEL LATERITE A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF APPLIED AND NATURAL SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY ECE FERİZOĞLU IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN METALLURGICAL AND MATERIALS ENGINEERING SEPTEMBER 2017 Approval of the thesis: SOLVENT EXTRACTION OF SCANDIUM FROM PREGNANT LEACH SOLUTION OF NICKEL LATERITES submitted by Ece FERİZOĞLU partial fulfillment of the requirements for the degree of Master of Science in Metallurgical and Materials Engineering Department, Middle East Technical University by, Prof. Dr. Gülbin Dural Ünver _________________ Dean, Graduate School of Natural and Applied Sciences Prof. Dr. Hakan Gür _________________ Head of Department, Metallurgical and Materials Engineering Prof. Dr. Rıza Gürbüz _________________ Supervisor, Metallurgical and Materials Engineering Dept., METU Prof. Dr. Yavuz A. Topkaya _________________ Co-Supervisor, Metallurgical and Materials Engineering Dept., METU Examining Committee Members: Prof. Dr. İshak Karakaya _________________ Metallurgical and Materials Engineering Dept., METU Metallurgical and Materials Engineering Dept., METU Prof. Dr. Rıza Gürbüz _________________ Prof. Dr. Yavuz A. Topkaya _________________ Metallurgical and Materials Engineering Dept., METU Assist. Prof. Dr. Metehan ERDOĞAN _________________ Metallurgical and Materials Engineering Dept., Yıldırım Beyazıt University Prof. Dr. Ümit Atalay _________________ Mining Engineering Dept., -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

And Ta-Bearing Oxide Minerals in the Greer Lake Pegmatitic Granite and Its Pegmatiteaureole, Southeasternmanitoba
American Mineralogist, Volume 71, pages 501-517, 1986 Fractionation trends of the Nb- and Ta-bearing oxide minerals in the Greer Lake pegmatitic granite and its pegmatiteaureole, southeasternManitoba Pnrn ennmi, Bnucn E. Golo,r FuNx C. H,lwruronNr, RoN CrHplr,lN Department of Earth Sciences,University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada Arsrucr The Greer Lake pegmatitic granite and related exterior rare-element pegmatites of the beryl-columbite type intrude metabasalt and tonalite gneiss in the Archean English River Subprovince of southeasternManitoba. Columbite-tantalite is the predominant Nb- and Ta-bearingmineral, associatedwith subordinateixiolite, microlite, niobian-tantalian rutile, and rare tantalian cassiterite, wodginite, and ilmenite. In coexisting mineral pairs, Tal(Ta + Nb) of microlite exceedsthat of columbite-tantalite, ixiolite, rutile, and cassiterite;in cassiteriteand rutile, Tal(Ta + Nb) is higher and Mn/(Mn * Fe) lower than in columbite- tantalite and ixiolite. In Li-, Rb-, Cs-, and F-poor environments, limited Mn enrichment accompaniesthe fractionation of Ta, which culminates in ixiolite and subordinate microlite. In Li-, Rb-, Cs-, and F-rich parageneses,extensive Mn enrichment precedesthe main Ta fractionation, which subsequentlygenerates near-end-member manganotantalite, wodginite, and mi- crolite. The Greer Lake and other fractionation trends indicate that a late-stageF-rich environment promotes extreme Fe/Mn fractionation prior to the main stage of Nb-Ta separation. The abundancesof Sn, Ti, and Sc are not related to fluorine or rare-alkali enrichment, but increasefrom pegmatitic granite to pegnatites. The relative accumulation of Ti and Sc in the pegmatite aureole seemsto be due to internal fractionation rather than assimilation. Columbite-tantalite in the pegmatitic granite shows an intermediate to near-ordered structural state, but is highly disordered in the pegmatite veins. -

Niobian Rutile from the Mcguire Granitic Pegmatite, Park County, Colorado: Solid Solution, Exsolution, and Oxidation
American Mineralogist, Volume 84, pages 754–763, 1999 Niobian rutile from the McGuire granitic pegmatite, Park County, Colorado: Solid solution, exsolution, and oxidation PETR Cˇ ERNY´ ,1,* RON CHAPMAN,1 WILLIAM B. SIMMONS,2 AND LEONARD E. CHACKOWSKY1 1 Department of Geological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada 2 Department of Geology and Geophysics, University of New Orleans, New Orleans, Louisiana 70148-2850, U.S.A. ABSTRACT Coarse crystals of niobian rutile occur in the hydrothermally altered core-margin zone of the McGuire granitic pegmatite, Park County, Colorado, associated with potassium feldspar, quartz, bi- otite, ilmenite, and monazite-(Ce). Primary homogeneous niobian rutile, with Fe3+ @ Fe2+ and a small excess of (Fe,Mn) over the amount required to compensate the incorporation of (Nb,Ta,W), under- went three stages of exsolution. Primary homogeneous niobian rutile exsolved a fine trellis-like pat- tern of minor lamellar Nb-bearing pseudorutile I. Most of this phase was broken down to pseudomor- phs consisting of microgranular Nb-rich pseudorutile II imbedded in niobian-ferrian “ferropseudobrookite.” Continued exsolution in niobian rutile and reconstitution of the early exsolution products generated (Fe,Nb)-depleted, microgranular niobian rutile, titanian ferrocolumbite, and mi- nor ilmenite. These three phases did not attain chemical equilibrium but may represent a stable phase assemblage. All these processes seem to have maintained charge balance, suggesting a closed sys- tem. Subsequent to the three stages of exsolution, extensive oxidation converted the mineral assem- blages to anatase + hematite + titanian-tungstenian ixiolite; primary ilmenite was oxidized into an anatase + hematite intergrowth. In both cases, the hematite component was almost completely leached out, leaving highly porous aggregates of the other phases. -

The Crystal Chemistry of the Tapiolite Series
631 The Canadian M ineralo g is t Vol. 34, pp.631,-647(1,996) THE CRYSTALCHEMISTRY OFTHE TAPIOLITE SERIES MICHAELA. WISE Departnunt of Mincral Sciences,Snr.ithsonian hstitution" Washington,D,C, 20560, U.SA. PETR dERNf Depamnentof GeologicalSciences, Unitersity of Maninba. Winnipeg,Manitoba R3T2N2 ABsrRAc"r Ferotapiolite-manganotapiolite-groupminslzls [@e,Mn)Ta2O6]axe formed as :rccessoryphases in rare-elementgranitic pegmatitesexhibiting moderateto high degreesof fractionation.Despite their relative paucity, 94 setsof unit-cell parameters and 194 chemical compositionswere compiled to characterizethe crystal chemistry of the tapiolite series.X-ray-diffraction studiesindicate that the degreeof orderand compositional variations stongly influenceunit-cell dimensionsof tapiolite.Natural tapiolite showswide rangesof structuralstate. Crystallization of disorderedphases at low lemperaturesis suggested;however, the available data are not unambiguous.Tapiolite chemistry is typically uniform (Fe > Mn, Ta > Nb), and compositional variations are the result of limited, but effective homovalentard heterovalentsubstitutions: (1) Mn2+Fe2+-r;(2) NbsiTas+-l; (3) Fek(ti,Sn)4rFe2+_r(IaM)5*-r and (4) @,sn)4,1Fe,Mn;{,6M,Ta)5*_2. ln association witl otler M,Ta,Sn-bearing minerals,tapiolite shows a distinct preferencefor Fd+ and Ta over Mn and Nb. Emicbment in Nb, Ti and Sn appearsto be commonin tapiolite from moderatelyfractionated pegmatites, whereas extreme Mn enrichmentis typical of highly fractionated pegmatitesof the petalite subtypeor metasomaticunits. Keywords:tapiolite series,crystal chemistry,order - disorder,tantalum" granitic pegmatite. SoM:uaRs Irs mindraux de la s6de ferrotapiolite - manganotapiolitetGe"IUn)TqO6l se pr6sententsous forme d'accessoiresdes pegmatitesgranitiques d 6l6mentsrares faisantpreuve d'un degr6de fractionnementintermddiaire tr 61ev6.Malgrd leur raret6, les r6sultatsde 94 affinementsde la maille 6l6mentaireet de 194 analyseschimiques sont disponibles,et serventi caract6riser la cristallochimie des min6raux de cette s6rie. -

Crystallization Processes and Solubility of Columbite-(Mn), Tantalite-(Mn), Microlite, Pyrochlore, Wodginite and Titanowodginite in Highly Fluxed Haplogranitic Melts
Western University Scholarship@Western Scholarship@Western Electronic Thesis and Dissertation Repository 3-12-2018 10:30 AM Crystallization processes and solubility of columbite-(Mn), tantalite-(Mn), microlite, pyrochlore, wodginite and titanowodginite in highly fluxed haplogranitic melts Alysha G. McNeil The University of Western Ontario Supervisor Linnen, Robert L. The University of Western Ontario Co-Supervisor Flemming, Roberta L. The University of Western Ontario Graduate Program in Geology A thesis submitted in partial fulfillment of the equirr ements for the degree in Doctor of Philosophy © Alysha G. McNeil 2018 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Earth Sciences Commons Recommended Citation McNeil, Alysha G., "Crystallization processes and solubility of columbite-(Mn), tantalite-(Mn), microlite, pyrochlore, wodginite and titanowodginite in highly fluxed haplogranitic melts" (2018). Electronic Thesis and Dissertation Repository. 5261. https://ir.lib.uwo.ca/etd/5261 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract Niobium and tantalum are critical metals that are necessary for many modern technologies such as smartphones, computers, cars, etc. Ore minerals of niobium and tantalum are typically associated with pegmatites and include columbite, tantalite, wodginite, titanowodginite, microlite and pyrochlore. Solubility and crystallization mechanisms of columbite-(Mn) and tantalite-(Mn) have been extensively studied in haplogranitic melts, with little research into other ore minerals. A new method of synthesis has been developed enabling synthesis of columbite-(Mn), tantalite-(Mn), hafnon, zircon, and titanowodginite for use in experiments at temperatures ≤ 850 °C and 200 MPa, conditions attainable by cold seal pressure vessels. -
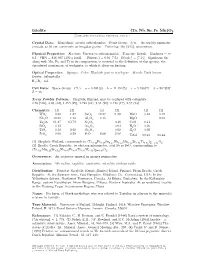
Ixiolite (Ta, Nb, Sn, Fe, Mn)O2 C 2001-2005 Mineral Data Publishing, Version 1
Ixiolite (Ta, Nb, Sn, Fe, Mn)O2 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic, pseudo-orthorhombic. Point Group: 2/m. As crudely prismatic crystals, to 10 cm; commonly as irregular grains. Twinning: On {013}, uncommon. Physical Properties: Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 6– 6.5 VHN = 836–967 (100 g load). D(meas.) = 6.94–7.23 D(calc.) = [7.34] Significant Sn, along with Mn, Fe, and Ti in its composition, is essential to the definition of this species, the disordered counterpart of wodginite, to which it alters on heating. Optical Properties: Opaque. Color: Blackish gray to steel-gray. Streak: Dark brown. Luster: Submetallic. R1–R2: n.d. Cell Data: Space Group: P 2/c. a = 9.481(3) b = 11.494(5) c = 5.158(2) β =90◦8(2)0 Z=16 X-ray Powder Pattern: Skogb¨ole,Finland; may be confused with columbite. 2.98 (100), 3.65 (32), 1.459 (29), 1.722 (24), 2.51 (20), 1.746 (17), 2.57 (13) Chemistry: (1) (2) (1) (2) (1) (2) WO3 0.30 1.87 SnO2 12.27 11.38 MnO 5.40 9.19 Nb2O5 10.50 6.12 Al2O3 0.16 MgO 0.01 Ta2O5 61.47 63.79 Sc2O3 0.16 CaO 0.11 + SiO2 0.12 As2O3 0.04 H2O 0.16 − TiO2 0.38 2.68 Sb2O3 0.02 H2O 0.08 ZrO2 0.60 0.20 FeO 8.08 2.98 Total 99.63 98.44 (1) Skogb¨ole,Finland; corresponds to (Ta0.44Fe0.18Sn0.13Nb0.12Mn0.12Zr0.01Ti0.01)Σ=1.01O2. -

Primary Minerals of the Jáchymov Ore District
Journal of the Czech Geological Society 48/34(2003) 19 Primary minerals of the Jáchymov ore district Primární minerály jáchymovského rudního revíru (237 figs, 160 tabs) PETR ONDRU1 FRANTIEK VESELOVSKÝ1 ANANDA GABAOVÁ1 JAN HLOUEK2 VLADIMÍR REIN3 IVAN VAVØÍN1 ROMAN SKÁLA1 JIØÍ SEJKORA4 MILAN DRÁBEK1 1 Czech Geological Survey, Klárov 3, CZ-118 21 Prague 1 2 U Roháèových kasáren 24, CZ-100 00 Prague 10 3 Institute of Rock Structure and Mechanics, V Holeovièkách 41, CZ-182 09, Prague 8 4 National Museum, Václavské námìstí 68, CZ-115 79, Prague 1 One hundred and seventeen primary mineral species are described and/or referenced. Approximately seventy primary minerals were known from the district before the present study. All known reliable data on the individual minerals from Jáchymov are presented. New and more complete X-ray powder diffraction data for argentopyrite, sternbergite, and an unusual (Co,Fe)-rammelsbergite are presented. The follow- ing chapters describe some unknown minerals, erroneously quoted minerals and imperfectly identified minerals. The present work increases the number of all identified, described and/or referenced minerals in the Jáchymov ore district to 384. Key words: primary minerals, XRD, microprobe, unit-cell parameters, Jáchymov. History of mineralogical research of the Jáchymov Chemical analyses ore district Polished sections were first studied under the micro- A systematic study of Jáchymov minerals commenced scope for the identification of minerals and definition early after World War II, during the period of 19471950. of their relations. Suitable sections were selected for This work was aimed at supporting uranium exploitation. electron microprobe (EMP) study and analyses, and in- However, due to the general political situation and the teresting domains were marked. -

Cation Distribution in Partially Ordered Columbite from the Kings Mountain
American Mineralogist, Volume 76, pages 1897-1904, 1991 Cation distribution in partially ordered columbite from the Kings Mountain pegmatite,North Carolina M,qnc WnNGrn.TrroNr.ts Anrvrnnusrnn I-aboratorium fiir chemischeund mineralogischeKristallographie, Universitit Bern, Freiestrasse3, CH-3012 Bern, Switzerland Cnlllns A. Gnrcnn BayerischesForschungsinstitut {iir experimentelle Geochemie und Geophysik, Universitdt Bayreuth, Postfach IOl25l, D-8580 Bayreuth, Germany ABSTRAcT Two columbite samples,space group Pbcn, with a : 14.233,b : 5.724,c : 5.104 A, Z: 4 and a: 14.221,b : 5.727,c : 5.102A, of similar chemistry(average composition FeourMno.roTi005Nb.TaorOu)but from different rock types of the Kings Mountain peg- matite, North Carolina, were investigated. One columbite specimen (NCP5) originates from a primary spodumene pegmatite, the other (NCPI) from a rock composed mainly of sugaryalbite formed by subsequenthydrothermal activity. Single-crystalX-ray structure refinements indicate that both columbite samplesshow strong disorder between (Nb,Ta) and (Fe,Mn) on octahedral A and B positions. This is in agreementwith a Mdssbauer spectrum (NCP5), which was fitted with two doublets characteristicof two distinct t61Fe2+ sites. Both samples show strong chemical zoning with respect to Nb and Ta, but weak zoning of Fe and Mn. The similar chemical composition and cation ordering indicate that columbite in both rocks is formed by the same process.Late hydrothermal alteration of the original spodumenepegmatite did not affect the composition of the earlier crystallized columbite. The primary columbite (NCP5) shows slightly stronger disorder of two- and five-valent cations than columbite (NCPI) found in the hydrothermally altered part of the pegmatite. INrnonucrroN some samplesreveal a wodginite pattern (Khvostova and Maximova, 1969),whereas others yield a columbite pat- Minerals of the columbite-tantalite group have the gen- tern (Nickel et al., 1963b). -

Mineralogy and Petrology of the Amazonite Pegmatite at Bakstevalåsen, Øvre Eiker
Master Thesis, Department of Geosciences Mineralogy and petrology of the amazonite pegmatite at Bakstevalåsen, øvre Eiker Øyvind Sunde Mineralogy and petrology of the amazonite pegmatite at Bakstevalåsen, øvre Eiker Øyvind Sunde Master Thesis in Geosciences Discipline: Geology Department of Geosciences Faculty of Mathematics and Natural Sciences University of Oslo July 2013 © Øyvind Sunde, 2013 Supervised by associate prof. Rune S. Selbekk and prof. Tom Andersen Cover picture: Hand specimen of the amazonite pegmatite at Bakstevalåsen measuring a 15 cm cross-section with amazonite matrix and abundant danalite. This work is published digitally through DUO – Digitale Utgivelser ved UiO http://www.duo.uio.no It is also catalogued in BIBSYS (http://www.bibsys.no/english) All rights reserved. No part of this publication may be reproduced or transmitted, in any form or by any means, without permission. Acknowledgements This thesis marks the end of a 5 –year period of time with relentless studies at the Department of Geosciences, University of Oslo. There are many people I have met during this 5-year ride who in various ways have contributed in shaping my interest for geology. I have never, ever, regretted my decision on setting sail onto this journey. You all know who you are and a huge thank you! My thesis would not have been possible without the help of several clever individuals, and I would like to aim a special appreciation to the following personnel: Rune Selbekk: first of all, thank you for letting me volunteer at the natural History Museum during my infant years of studying. It brought more geology into a curriculum diluted with meteorology and philosophy. -

Shin-Skinner January 2018 Edition
Page 1 The Shin-Skinner News Vol 57, No 1; January 2018 Che-Hanna Rock & Mineral Club, Inc. P.O. Box 142, Sayre PA 18840-0142 PURPOSE: The club was organized in 1962 in Sayre, PA OFFICERS to assemble for the purpose of studying and collecting rock, President: Bob McGuire [email protected] mineral, fossil, and shell specimens, and to develop skills in Vice-Pres: Ted Rieth [email protected] the lapidary arts. We are members of the Eastern Acting Secretary: JoAnn McGuire [email protected] Federation of Mineralogical & Lapidary Societies (EFMLS) Treasurer & member chair: Trish Benish and the American Federation of Mineralogical Societies [email protected] (AFMS). Immed. Past Pres. Inga Wells [email protected] DUES are payable to the treasurer BY January 1st of each year. After that date membership will be terminated. Make BOARD meetings are held at 6PM on odd-numbered checks payable to Che-Hanna Rock & Mineral Club, Inc. as months unless special meetings are called by the follows: $12.00 for Family; $8.00 for Subscribing Patron; president. $8.00 for Individual and Junior members (under age 17) not BOARD MEMBERS: covered by a family membership. Bruce Benish, Jeff Benish, Mary Walter MEETINGS are held at the Sayre High School (on Lockhart APPOINTED Street) at 7:00 PM in the cafeteria, the 2nd Wednesday Programs: Ted Rieth [email protected] each month, except JUNE, JULY, AUGUST, and Publicity: Hazel Remaley 570-888-7544 DECEMBER. Those meetings and events (and any [email protected] changes) will be announced in this newsletter, with location Editor: David Dick and schedule, as well as on our website [email protected] chehannarocks.com. -

Geochemistry of Cassiterite and Wolframite from Tin and Tungsten Quartz Veins in Portugal ⁎ A.M.R
Available online at www.sciencedirect.com Ore Geology Reviews 33 (2008) 221–238 www.elsevier.com/locate/oregeorev Geochemistry of cassiterite and wolframite from tin and tungsten quartz veins in Portugal ⁎ A.M.R. Neiva Department of Earth Sciences, University of Coimbra, 3000-272 Coimbra, Portugal Received 9 August 2005; accepted 29 May 2006 Available online 4 October 2007 Abstract Cassiterite and wolframite compositions from SnNW- and WNSn-bearing quartz veins in Northern and Central Portugal are compared to provide evidence on fluid compositions. In SnNW-bearing quartz veins, euhedral cassiterite shows sequences of alternating parallel darker and lighter zones. The darker zones are pleochroic, oscillatory zoned, exhibit exsolutions of columbite and ixiolite and are richer in Nb, Ta and Fe than the lighter zones which consist of nearly pure SnO2. Cassiterite from WNSn-bearing quartz veins is usually zoned, with homogeneous and slightly pleochroic darker zones, which are chemically similar to lighter zones. Both zones have inclusions of rutile and rare ilmenite. The darker zones of cassiterite from the former veins are richer in Nb, Ta and Fe contents and poorer in Ti than the darker and lighter zones of cassiterite from the latter veins. This is attributed to differences in the composition of magmatic hydrothermal fluids. Wolframite compositions from SnNW- and W NSn-bearing quartz veins do not show any significant distinction, because they precipitate from relatively similar magmatic hydrothermal fluids. In some deposits, most wolframite crystals are homogeneous, but others are heterogeneous. Inner patches, rich in a hübnerite component, rarely occur in crystals from the Filharoso and Panasqueira deposits.