Cloning of the ALG8 Locus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Congenital Disorders of Glycosylation from a Neurological Perspective
brain sciences Review Congenital Disorders of Glycosylation from a Neurological Perspective Justyna Paprocka 1,* , Aleksandra Jezela-Stanek 2 , Anna Tylki-Szyma´nska 3 and Stephanie Grunewald 4 1 Department of Pediatric Neurology, Faculty of Medical Science in Katowice, Medical University of Silesia, 40-752 Katowice, Poland 2 Department of Genetics and Clinical Immunology, National Institute of Tuberculosis and Lung Diseases, 01-138 Warsaw, Poland; [email protected] 3 Department of Pediatrics, Nutrition and Metabolic Diseases, The Children’s Memorial Health Institute, W 04-730 Warsaw, Poland; [email protected] 4 NIHR Biomedical Research Center (BRC), Metabolic Unit, Great Ormond Street Hospital and Institute of Child Health, University College London, London SE1 9RT, UK; [email protected] * Correspondence: [email protected]; Tel.: +48-606-415-888 Abstract: Most plasma proteins, cell membrane proteins and other proteins are glycoproteins with sugar chains attached to the polypeptide-glycans. Glycosylation is the main element of the post- translational transformation of most human proteins. Since glycosylation processes are necessary for many different biological processes, patients present a diverse spectrum of phenotypes and severity of symptoms. The most frequently observed neurological symptoms in congenital disorders of glycosylation (CDG) are: epilepsy, intellectual disability, myopathies, neuropathies and stroke-like episodes. Epilepsy is seen in many CDG subtypes and particularly present in the case of mutations -

Biomedical Applications of Bacteria-Derived Polymers
polymers Review Biomedical Applications of Bacteria-Derived Polymers Jonathan David Hinchliffe, Alakananda Parassini Madappura, Syed Mohammad Daniel Syed Mohamed and Ipsita Roy * Department of Materials Science and Engineering, Faculty of Engineering, University of Sheffield, Sheffield S1 3JD, UK; jhinchliffe3@sheffield.ac.uk (J.D.H.); [email protected] (A.P.M.); smdsyedmohamed1@sheffield.ac.uk (S.M.D.S.M.) * Correspondence: I.Roy@sheffield.ac.uk; Tel.: +44-11-4222-5962 Abstract: Plastics have found widespread use in the fields of cosmetic, engineering, and medical sciences due to their wide-ranging mechanical and physical properties, as well as suitability in biomedical applications. However, in the light of the environmental cost of further upscaling current methods of synthesizing many plastics, work has recently focused on the manufacture of these polymers using biological methods (often bacterial fermentation), which brings with them the advantages of both low temperature synthesis and a reduced reliance on potentially toxic and non-eco-friendly compounds. This can be seen as a boon in the biomaterials industry, where there is a need for highly bespoke, biocompatible, processable polymers with unique biological properties, for the regeneration and replacement of a large number of tissue types, following disease. However, barriers still remain to the mass-production of some of these polymers, necessitating new research. This review attempts a critical analysis of the contemporary literature concerning the use of a number of bacteria-derived polymers in the context of biomedical applications, including the biosynthetic Citation: Hinchliffe, J.D.; Parassini pathways and organisms involved, as well as the challenges surrounding their mass production. -

Viruses Like Sugars: How to Assess Glycan Involvement in Viral Attachment
microorganisms Review Viruses Like Sugars: How to Assess Glycan Involvement in Viral Attachment Gregory Mathez and Valeria Cagno * Institute of Microbiology, Lausanne University Hospital, University of Lausanne, 1011 Lausanne, Switzerland; [email protected] * Correspondence: [email protected] Abstract: The first step of viral infection requires interaction with the host cell. Before finding the specific receptor that triggers entry, the majority of viruses interact with the glycocalyx. Identifying the carbohydrates that are specifically recognized by different viruses is important both for assessing the cellular tropism and for identifying new antiviral targets. Advances in the tools available for studying glycan–protein interactions have made it possible to identify them more rapidly; however, it is important to recognize the limitations of these methods in order to draw relevant conclusions. Here, we review different techniques: genetic screening, glycan arrays, enzymatic and pharmacological approaches, and surface plasmon resonance. We then detail the glycan interactions of enterovirus D68 and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), highlighting the aspects that need further clarification. Keywords: attachment receptor; viruses; glycan; sialic acid; heparan sulfate; HBGA; SARS-CoV-2; EV-D68 Citation: Mathez, G.; Cagno, V. Viruses Like Sugars: How to Assess 1. Introduction Glycan Involvement in Viral This review focuses on methods for assessing the involvement of carbohydrates in Attachment. Microorganisms 2021, 9, viral attachment and entry into the host cell. Viruses often bind to entry receptors that are 1238. https://doi.org/10.3390/ not abundant on the cell surface; to increase their chances of finding them, they initially microorganisms9061238 bind to attachment receptors comprising carbohydrates that are more widely expressed. -

Prenatal Testing Requisition Form
BAYLOR MIRACA GENETICS LABORATORIES SHIP TO: Baylor Miraca Genetics Laboratories 2450 Holcombe, Grand Blvd. -Receiving Dock PHONE: 800-411-GENE | FAX: 713-798-2787 | www.bmgl.com Houston, TX 77021-2024 Phone: 713-798-6555 PRENATAL COMPREHENSIVE REQUISITION FORM PATIENT INFORMATION NAME (LAST,FIRST, MI): DATE OF BIRTH (MM/DD/YY): HOSPITAL#: ACCESSION#: REPORTING INFORMATION ADDITIONAL PROFESSIONAL REPORT RECIPIENTS PHYSICIAN: NAME: INSTITUTION: PHONE: FAX: PHONE: FAX: NAME: EMAIL (INTERNATIONAL CLIENT REQUIREMENT): PHONE: FAX: SAMPLE INFORMATION CLINICAL INDICATION FETAL SPECIMEN TYPE Pregnancy at risk for specific genetic disorder DATE OF COLLECTION: (Complete FAMILIAL MUTATION information below) Amniotic Fluid: cc AMA PERFORMING PHYSICIAN: CVS: mg TA TC Abnormal Maternal Screen: Fetal Blood: cc GESTATIONAL AGE (GA) Calculation for AF-AFP* NTD TRI 21 TRI 18 Other: SELECT ONLY ONE: Abnormal NIPT (attach report): POC/Fetal Tissue, Type: TRI 21 TRI 13 TRI 18 Other: Cultured Amniocytes U/S DATE (MM/DD/YY): Abnormal U/S (SPECIFY): Cultured CVS GA ON U/S DATE: WKS DAYS PARENTAL BLOODS - REQUIRED FOR CMA -OR- Maternal Blood Date of Collection: Multiple Pregnancy Losses LMP DATE (MM/DD/YY): Parental Concern Paternal Blood Date of Collection: Other Indication (DETAIL AND ATTACH REPORT): *Important: U/S dating will be used if no selection is made. Name: Note: Results will differ depending on method checked. Last Name First Name U/S dating increases overall screening performance. Date of Birth: KNOWN FAMILIAL MUTATION/DISORDER SPECIFIC PRENATAL TESTING Notice: Prior to ordering testing for any of the disorders listed, you must call the lab and discuss the clinical history and sample requirements with a genetic counselor. -

F04b57ca351b0dc3389ebe992c
Review Insights into complexity of congenital disorders of glycosylation Sandra Supraha Goreta*, Sanja Dabelic, Jerka Dumic University of Zagreb, Faculty of Pharmacy and Biochemistry, Department of Biochemistry and Molecular Biology, Zagreb, Croatia *Corresponding author: [email protected] Abstract Biochemical and biological properties of glycoconjugates are strongly determined by the specifi c structure of its glycan parts. Glycosylation, the covalent attachment of sugars to proteins and lipids, is very complex and highly-coordinated process involving > 250 gene products. Defi ciency of glycosylation enzymes or transporters results in impaired glycosylation, and consequently pathological modulation of many physiological processes. Inborn defects of glycosylation enzymes, caused by the specifi cmutations, lead to the development of rare, but severe diseases – congenital disor- ders of glycosylation (CDGs). Up today, there are more than 45 known CDGs. Their clinical manifestations range from very mild to extremely severe (even lethal) and unfortunately, only three of them can be eff ectively treated nowadays. CDG symptoms highly vary, though someare common for several CDG types but also for other unrelated diseases, especially neurological ones, leaving the possibility that many CDGs cases are under- or mis- diagnosed. Glycan analysis of serum transferrin (by isoelectric focusing or more sophisticated methods, such as HPLC (high-performance liquid chro- matography) or MALDI (matrix-assisted laser desorption/ionization)) or serum N-glycans -

Metabolic Enzyme Expression Highlights a Key Role for MTHFD2 and the Mitochondrial Folate Pathway in Cancer
ARTICLE Received 1 Nov 2013 | Accepted 17 Dec 2013 | Published 23 Jan 2014 DOI: 10.1038/ncomms4128 Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer Roland Nilsson1,2,*, Mohit Jain3,4,5,6,*,w, Nikhil Madhusudhan3,4,5, Nina Gustafsson Sheppard1,2, Laura Strittmatter3,4,5, Caroline Kampf7, Jenny Huang8, Anna Asplund7 & Vamsi K. Mootha3,4,5 Metabolic remodeling is now widely regarded as a hallmark of cancer, but it is not clear whether individual metabolic strategies are frequently exploited by many tumours. Here we compare messenger RNA profiles of 1,454 metabolic enzymes across 1,981 tumours spanning 19 cancer types to identify enzymes that are consistently differentially expressed. Our meta- analysis recovers established targets of some of the most widely used chemotherapeutics, including dihydrofolate reductase, thymidylate synthase and ribonucleotide reductase, while also spotlighting new enzymes, such as the mitochondrial proline biosynthetic enzyme PYCR1. The highest scoring pathway is mitochondrial one-carbon metabolism and is centred on MTHFD2. MTHFD2 RNA and protein are markedly elevated in many cancers and correlated with poor survival in breast cancer. MTHFD2 is expressed in the developing embryo, but is absent in most healthy adult tissues, even those that are proliferating. Our study highlights the importance of mitochondrial compartmentalization of one-carbon metabolism in cancer and raises important therapeutic hypotheses. 1 Unit of Computational Medicine, Department of Medicine, Karolinska Institutet, 17176 Stockholm, Sweden. 2 Center for Molecular Medicine, Karolinska Institutet, 17176 Stockholm, Sweden. 3 Broad Institute, Cambridge, Massachusetts 02142, USA. 4 Department of Systems Biology, Harvard Medical School, Boston, Massachusetts 02115, USA. -

Genome-Wide Investigation of Cellular Functions for Trna Nucleus
Genome-wide Investigation of Cellular Functions for tRNA Nucleus- Cytoplasm Trafficking in the Yeast Saccharomyces cerevisiae DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Hui-Yi Chu Graduate Program in Molecular, Cellular and Developmental Biology The Ohio State University 2012 Dissertation Committee: Anita K. Hopper, Advisor Stephen Osmani Kurt Fredrick Jane Jackman Copyright by Hui-Yi Chu 2012 Abstract In eukaryotic cells tRNAs are transcribed in the nucleus and exported to the cytoplasm for their essential role in protein synthesis. This export event was thought to be unidirectional. Surprisingly, several lines of evidence showed that mature cytoplasmic tRNAs shuttle between nucleus and cytoplasm and their distribution is nutrient-dependent. This newly discovered tRNA retrograde process is conserved from yeast to vertebrates. Although how exactly the tRNA nuclear-cytoplasmic trafficking is regulated is still under investigation, previous studies identified several transporters involved in tRNA subcellular dynamics. At least three members of the β-importin family function in tRNA nuclear-cytoplasmic intracellular movement: (1) Los1 functions in both the tRNA primary export and re-export processes; (2) Mtr10, directly or indirectly, is responsible for the constitutive retrograde import of cytoplasmic tRNA to the nucleus; (3) Msn5 functions solely in the re-export process. In this thesis I focus on the physiological role(s) of the tRNA nuclear retrograde pathway. One possibility is that nuclear accumulation of cytoplasmic tRNA serves to modulate translation of particular transcripts. To test this hypothesis, I compared expression profiles from non-translating mRNAs and polyribosome-bound translating mRNAs collected from msn5Δ and mtr10Δ mutants and wild-type cells, in fed or acute amino acid starvation conditions. -

CDG-Ih) (ALG8 Deficiency
550 LETTER TO JMG J Med Genet: first published as 10.1136/jmg.2003.016923 on 2 July 2004. Downloaded from Clinical and molecular features of three patients with congenital disorders of glycosylation type Ih (CDG-Ih) (ALG8 deficiency) E Schollen, C G Frank, L Keldermans, R Reyntjens, C E Grubenmann, P T Clayton, B G Winchester, J Smeitink, R A Wevers, M Aebi, T Hennet, G Matthijs ............................................................................................................................... J Med Genet 2004;41:550–556. doi: 10.1136/jmg.2003.016923 rotein glycosylation is an essential post-translational Key points modification of various proteins, affecting their folding, Psorting, and function. Inborn defects in the assembly and processing of glycans on glycoproteins are known as N Congenital disorder of glycosylation type Ih (CDG-Ih) congenital disorders of glycosylation (CDG) and can affect is caused by a defect in the dolichyl-P-Glc:Glc1 both N- and O-glycosylation (for reviews see Marquardt and Man9GlcNAc2-PP-dolichyl a1,3-glucosyltransferase Denecke,1 Jaeken,2 and Grunewald et al3). N-glycosylation (ALG8). Thus far, the only patient has been described defects and especially defects in the assembly of the dolichol with a relatively mild clinical picture. linked N-glycan precursor in the endoplasmic reticulum (ER) N We describe the molecular and clinical features of (CDG-I) result in hypoglycosylation of many kinds of three patients from two families with an ALG8 (serum) glycoproteins. CDG-I is therefore a group of multi- deficiency. All three patients presented with severe, systemic disorders. life-threatening multi organ failure and died within The assembly of the N-glycan precursor in the ER is a their first months of life. -
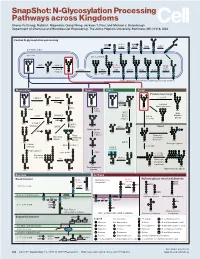
N-Glycosylation Processing Pathways Across Kingdoms Cheng-Yu Chung, Natalia I
SnapShot: N-Glycosylation Processing Pathways across Kingdoms Cheng-Yu Chung, Natalia I. Majewska, Qiong Wang, Jackson T. Paul, and Michael J. Betenbaugh Department of Chemical and Biomolecular Engineering, The Johns Hopkins University, Baltimore, MD 21218, USA Central N-glycosylation processing UDP ALG13 GDP ALG7 ALG14 ALG1 ALG2 ALG11 P P P P P CYTOPLASM P P P P P P GOLGI ER LUMEN GNTI α -Man IA En Bloc ALG3 Transfer ALG9 α-Man IB ER Man I α -Glc I OST ALG10 ALG8 ALG6 ALG12 Asn α -Man IC Asn Asn Asn α -Glc II P P P P P Asn P P P P P ALGs: asparagine-linked N-glycosylation processing enzymes Mammalian Insect Plant Fungi Filamentous fungi α-Man II FUT8 α-1,2-MT XYLT GDP Terminal Asn Asn Asn Asn UDP Asn Galactofuranose GNTII Asn Asn FUT8 f ± α-Gal Asn FUTC3 GNTII High FUT11 α -GALT B14GALTI Och1p Mannose FUT12 glycan α-Man II α-1,2-MT UDP Asn Asn ST3GAL4 Asn Asn GNTIV ST6GAL1 GNTIII GNTV Asn GNase Yeast Hybrid glycan P CMP GNase Asn Asn Tetra- α-Man II Mannosyl- Bisecting antennary phosphate glycan glycan transferase Asn Asn Asn Asn GALT1 Asn ST8SIA2 Core type glycan GNTs B14GALTI α-1,6-MT ST8SIA4 Asn Lewis A structure Polysialylation α-Man PolyLacNAc α-1,2-MT Alternative α-1,3-MT P sialic acid FUT13 ] [ STs Mannosyl- n phosphate Asn Asn Asn transferase Asn Asn Paucimannose Hypermannose glycan Asn Asn Asn glycans Bacteria Archaea Archaea glycan structural diversity Block transfer En Bloc Methanococcus En Bloc Transfer Transfer maripaludis OCH3 Thr OCH AgIB Thr OCH3 3 PERIPLASM PglB B B Asn P - P Asn OSO3 P Asn Asn INNER MEMBRANE P Asn P -

Clinical, Molecular, and Immune Analysis of Dabrafenib-Trametinib
Supplementary Online Content Chen G, McQuade JL, Panka DJ, et al. Clinical, molecular and immune analysis of dabrafenib-trametinib combination treatment for metastatic melanoma that progressed during BRAF inhibitor monotherapy: a phase 2 clinical trial. JAMA Oncology. Published online April 28, 2016. doi:10.1001/jamaoncol.2016.0509. eMethods. eReferences. eTable 1. Clinical efficacy eTable 2. Adverse events eTable 3. Correlation of baseline patient characteristics with treatment outcomes eTable 4. Patient responses and baseline IHC results eFigure 1. Kaplan-Meier analysis of overall survival eFigure 2. Correlation between IHC and RNAseq results eFigure 3. pPRAS40 expression and PFS eFigure 4. Baseline and treatment-induced changes in immune infiltrates eFigure 5. PD-L1 expression eTable 5. Nonsynonymous mutations detected by WES in baseline tumors This supplementary material has been provided by the authors to give readers additional information about their work. © 2016 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/30/2021 eMethods Whole exome sequencing Whole exome capture libraries for both tumor and normal samples were constructed using 100ng genomic DNA input and following the protocol as described by Fisher et al.,3 with the following adapter modification: Illumina paired end adapters were replaced with palindromic forked adapters with unique 8 base index sequences embedded within the adapter. In-solution hybrid selection was performed using the Illumina Rapid Capture Exome enrichment kit with 38Mb target territory (29Mb baited). The targeted region includes 98.3% of the intervals in the Refseq exome database. Dual-indexed libraries were pooled into groups of up to 96 samples prior to hybridization. -

Structural and Biochemical Insights Into Biosynthesis and Degradation of and Degradation Into Insights Biosynthesis and Biochemical Structural
Tom Reichenbach Tom kth royal institute of technology Structuralbiochemicaland biosynthesisinsights into degradation and of Doctoral Thesis in Biotechnology Structural and biochemical insights into biosynthesis and degradation of N-glycans TOM REICHENBACH N -glycans ISBN 978-91-7873-660-7 TRITA-CBH-FOU-2020:41 KTH2020 www.kth.se Stockholm, Sweden 2020 Structural and biochemical insights into biosynthesis and degradation of N-glycans TOM REICHENBACH Academic Dissertation which, with due permission of the KTH Royal Institute of Technology, is submitted for public defence for the Degree of Doctor of Philosophy on Friday the 16th October 2020, at 10:00 a.m. in Kollegiesalen, KTH, Brinellvägen 8, Stockholm. Doctoral Thesis in Biotechnology KTH Royal Institute of Technology Stockholm, Sweden 2020 © Tom Reichenbach ISBN 978-91-7873-660-7 TRITA-CBH-FOU-2020:41 Printed by: Universitetsservice US-AB, Sweden 2020 Abstract Carbohydrates are a primary energy source for all living organisms, but importantly, they also participate in a number of life-sustaining biological processes, e.g. cell signaling and cell-wall synthesis. The first part of the thesis examines glycosyltransferases that play a crucial role in the biosynthesis of N-glycans. Precursors to eukaryotic N-glycans are synthesized in the endoplasmic reticulum (ER) in the form of a lipid-bound oligosaccharide, which is then transferred to a nascent protein. The first seven sugar units are assembled on the cytoplasmic side of the ER, which is performed by glycosyltransferases that use nucleotide sugars as donors. The mannosyl transferase PcManGT is produced by the archaeon Pyrobaculum calidifontis, and the biochemical and structural results presented in the thesis suggest that the enzyme may be a counterpart to the glycosyltransferase Alg1 that participates in the biosynthesis of N-glycans in eukaryotes. -

SI Correction
SI Correction SYSTEMS BIOLOGY Correction to Supporting Information for “miRNA proxy approach issue 23, June 9, 2015, of Proc Natl Acad Sci USA (112:7327–7332; reveals hidden functions of glycosylation,” by Tomasz Kurcon, first published May 26, 2015; 10.1073/pnas.1502076112). Zhongyin Liu, Anika V. Paradkar, Christopher A. Vaiana, Sujeethraj The authors note that Table S1, Table S3, and Table S4 ap- Koppolu, Praveen Agrawal, and Lara K. Mahal, which appeared in peared incorrectly. The SI has been corrected online. Table S1. Classification of miR-200f predicted glycogenes according to the type of glycan synthesized (related to Table S3) N-linked O-linked NC-O-linked GAGs Glycolipid Other/terminal ALG2 C1GALT1 B3GAT1 CSGALNACT2 B3GALT2 B3GALNT2 ALG5 GALNT2 B3GLCT B3GALT6 B3GALNT1 B3GNT1 ALG6 GALNT3 GXYLT1 CHST2 B3GNT4 B3GNT2 ALG8 GALNT4 LARGE CHST7 B3GNT5 FUT2 ALG11 GALNT10 LFNG CHST12 B4GALT6 FUT4 ALG14 GALNT11 OGT CHSY1 GAL3ST1 HAS2 DDOST GALNT12 POFUT1 CHSY3 ST3GAL5 HAS3 DPAGT1 GALNT14 EXTL2 ST3GAL6 ST3GAL2 MGAT2 GALNTL2 HS2ST1 ST6GALNAC3 ST3GAL3 MGAT3 GCNT2 HS3ST1 ST6GALNAC5 ST8SIA2 MGAT4A GCNT4 HS3ST3A1 UGCG ST8SIA4 MGAT4C HS3ST5 HS6ST1 HS6ST2 HS6ST3 NDST3 Count 12 11 7 16 11 11 % Total 17.6 16.2 10.3 23.5 16.2 16.2 miR-200f predicted glycogenes were classified according to the glycan they synthesize using the Kyoto Encyclopedia of Genes and Genomes. The % total represents the distribution of one type of glycan among all predicted targets. GAGs, glycosaminoglycans; NC O-linked, noncanonical O-linked. E4632–E4635 | PNAS | August 18, 2015 | vol. 112 | no. 33 www.pnas.org Downloaded by guest on September 28, 2021 Table S3.