Trophic Interactions and Ecosystem Management at the New Zealand Subantarctic Islands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proposal for Inclusion of the Antipodean Albatross in Appendix I
CONVENTION ON UNEP/CMS/COP13/Doc. 27.1.7 MIGRATORY 25 September 2019 Original: English SPECIES 13th MEETING OF THE CONFERENCE OF THE PARTIES Gandhinagar, India, 17 - 22 February 2020 Agenda Item 27.1 PROPOSAL FOR THE INCLUSION OF THE ANTIPODEAN ALBATROSS (Diomedea antipodensis) ON APPENDIX I OF THE CONVENTION Summary: The Governments of New Zealand, Australia and Chile have submitted the attached proposal for the inclusion of the Antipodean albatross (Diomedea antipodensis) on Appendix I of CMS. The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CMS Secretariat (or the United Nations Environment Programme) concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. UNEP/CMS/COP13/Doc. 27.1.7 PROPOSAL FOR INCLUSION OF THE ANTIPODEAN ALBATROSS (Diomedea antipodensis) ON APPENDIX I OF THE CONVENTION A. PROPOSAL Inclusion of Diomedea antipodensis on the Convention on the Conservation of Migratory Species of Wild Animals (CMS) Appendix I. The current CMS Appendix II listing will remain in place. Diomedea antipodensis is classified as Endangered (IUCN) as it is undergoing a very rapid decline in population size. B. PROPONENT: Governments of New Zealand, Australia and Chile. C. SUPPORTING STATEMENT 1. Taxonomy 1.1 Class: Aves 1.2 Order: Procellariiformes 1.3 Family: Diomedeidae (albatrosses) 1.4 Genus, species or subspecies, including author and year: Diomedea antipodensis (Robertson & Warham 1992), including two subspecies: Diomedea antipodensis antipodensis and Diomedea antipodensis gibsoni 1.5 Scientific synonyms: Diomedea exulans antipodensis Diomedea antipodensis was formerly included in the wandering albatross complex (Diomedea exulans) (e.g. -

TAG Operational Structure
PARROT TAXON ADVISORY GROUP (TAG) Regional Collection Plan 5th Edition 2020-2025 Sustainability of Parrot Populations in AZA Facilities ...................................................................... 1 Mission/Objectives/Strategies......................................................................................................... 2 TAG Operational Structure .............................................................................................................. 3 Steering Committee .................................................................................................................... 3 TAG Advisors ............................................................................................................................... 4 SSP Coordinators ......................................................................................................................... 5 Hot Topics: TAG Recommendations ................................................................................................ 8 Parrots as Ambassador Animals .................................................................................................. 9 Interactive Aviaries Housing Psittaciformes .............................................................................. 10 Private Aviculture ...................................................................................................................... 13 Communication ........................................................................................................................ -

Andrea Milković New Zealand and Its Tourism Potential
New Zealand and its Tourism Potential Milković, Andrea Undergraduate thesis / Završni rad 2017 Degree Grantor / Ustanova koja je dodijelila akademski / stručni stupanj: Polytechnic of Međimurje in Čakovec / Međimursko veleučilište u Čakovcu Permanent link / Trajna poveznica: https://urn.nsk.hr/urn:nbn:hr:110:471894 Rights / Prava: In copyright Download date / Datum preuzimanja: 2021-09-30 Repository / Repozitorij: Polytechnic of Međimurje in Čakovec Repository - Polytechnic of Međimurje Undergraduate and Graduate Theses Repository MEĐIMURSKO VELEUČILIŠTE U ČAKOVCU STRUČNI STUDIJ MENADŢMENT TURIZMA I SPORTA ANDREA MILKOVIĆ NEW ZEALAND AND ITS TOURISM POTENTIAL ZAVRŠNI RAD ČAKOVEC, 2016. POLYTECHNIC OF MEĐIMURJE IN ČAKOVEC PROFESSIONAL STUDY PROGRAME MANAGEMENT OF TOURISM AND SPORT ANDREA MILKOVIĆ NEW ZEALAND AND ITS TOURISM POTENTIAL FINAL PAPER Mentor: Marija Miščančuk, prof. ČAKOVEC, 2016 Zahvala: Veliku zahvalnost, u prvom redu, dugujem svojoj mentorici, prof. Mariji Miščančuk zbog savjetovanja, usmjeravanja i odvojenog vremena tijekom pisanja ovog završnog rada. Zahvaljujem se i ostalim djelatnicima na MeĎimurskom Veleučilištu u Čakovcu zbog kvalitetnog prenošenja znanja i pomoći tijekom studiranja. Veliko hvala Antoniju Kovačeviću i sestri Nikolini Milković na pomoći oko nabavljanja literature i tehničkoj podršci. Isto tako, zahvaljujem im se na ohrabrenju i moralnoj podršci za vrijeme pisanja rada, ali i tijekom cijelog studiranja. TakoĎer, hvala mojim prijateljima Goranu Haramasu, Martini Šestak, Petri Benotić, Petri Kozulić i Vinki Kugelman koji su bili uz mene i učinili ove studijske godine ljepšima. Hvala mojoj obitelji na podršci i strpljenju tokom studija. ABSTRACT Curiosity of people leads to traveling for pleasure to new places where they can visit and learn about historical buildings, natural beauty and anything that makes one country special, interesting and worth visiting. -

Aspects of the Ecology of Antipodes Island Parakeet ( Cyanoramphus Unicolor) and Reischek's Parakeet ( C
Aspects of the ecology of Antipodes Island Parakeet ( Cyanoramphus unicolor) and Reischek's Parakeet ( C. novaezelandiae hochstetten) on Antipodes Island, October - November 1995 TERRY C. GREENE Science and Research Unit, Department of Conservation, Private Bag 68-908, Newton, Auckland, New Zealad ABSTRACT Aspects of the ecology of the Antipodes Island Parakeet (Cyanoramphus unicolor) and Reischek's Parakeet (C. novaezelandiae hochstettq were examined at the Antipodes Islands during October and November 1995. Significant differences in diet were detected between the species. Leaves of large tussocks formed the majority of the diet forhtipodes Island Parakeets, whereas tussock flowers comprised much of the diet of Reischek's Parakeet. Significant differences in the diet of these species between this and previous studies were thought to reflect temporal variations in food availability rather than any fundamental shift in dietary preferences. Observations were made of both parakeet species scavenging on bird corpses. Antipodes Island Parakeets were also recorded killing and eating Grey-backed Storm Petrels (Oceanites nereis). One Antipodes Island Parakeet nest was located; clutch size, physical characteristics of the nest and of behaviour during incubation are described. KEYWORDS: Antipodes Island Parakeet, Cyanorampbus unicolor, Reischek's Parakeet, Cyanoramphus novaezelandiae hochstetteri, Antipodes Islands, diet, predation INTRODUCTION Two parakeet species of the genus Cyanorampbus (subfamily: Psittacinae) occur sympatrically on the subantarctic Antipodes Islands. The parakeets form a distinct and significant part of an avifauna otherwise dominated by seabirds. The Antipodes Island Parakeet (Cyanoramphus unicolor) is the largest species of its genus and is endemic to the island group. Reischek's Parakeet (C. novaezelandiae hochstetteri) is also endemic to the Antipodes Islands, and is one of four other subspecies of the nominate Red-crowned Parakeet (C. -

New Zealand Subantarctic Islands Research Strategy
New Zealand Subantarctic Islands Research Strategy SOUTHLAND CONSERVANCY New Zealand Subantarctic Islands Research Strategy Carol West MAY 2005 Cover photo: Recording and conservation treatment of Butterfield Point fingerpost, Enderby Island, Auckland Islands Published by Department of Conservation PO Box 743 Invercargill, New Zealand. CONTENTS Foreword 5 1.0 Introduction 6 1.1 Setting 6 1.2 Legal status 8 1.3 Management 8 2.0 Purpose of this research strategy 11 2.1 Links to other strategies 12 2.2 Monitoring 12 2.3 Bibliographic database 13 3.0 Research evaluation and conditions 14 3.1 Research of benefit to management of the Subantarctic islands 14 3.2 Framework for evaluation of research proposals 15 3.2.1 Research criteria 15 3.2.2 Risk Assessment 15 3.2.3 Additional points to consider 16 3.2.4 Process for proposal evaluation 16 3.3 Obligations of researchers 17 4.0 Research themes 18 4.1 Theme 1 – Natural ecosystems 18 4.1.1 Key research topics 19 4.1.1.1 Ecosystem dynamics 19 4.1.1.2 Population ecology 20 4.1.1.3 Disease 20 4.1.1.4 Systematics 21 4.1.1.5 Biogeography 21 4.1.1.6 Physiology 21 4.1.1.7 Pedology 21 4.2 Theme 2 – Effects of introduced biota 22 4.2.1 Key research topics 22 4.2.1.1 Effects of introduced animals 22 4.2.1.2 Effects of introduced plants 23 4.2.1.3 Exotic biota as agents of disease transmission 23 4.2.1.4 Eradication of introduced biota 23 4.3 Theme 3 – Human impacts and social interaction 23 4.3.1 Key research topics 24 4.3.1.1 History and archaeology 24 4.3.1.2 Human interactions with wildlife 25 4.3.1.3 -

Engelsk Register
Danske navne på alverdens FUGLE ENGELSK REGISTER 1 Bearbejdning af paginering og sortering af registret er foretaget ved hjælp af Microsoft Excel, hvor det har været nødvendigt at indlede sidehenvisningerne med et bogstav og eventuelt 0 for siderne 1 til 99. Tallet efter bindestregen giver artens rækkefølge på siden. -

Northern Giant Petrel Macronectes Halli Breeding Population Survey, Auckland Islands
Northern giant petrel Macronectes halli breeding population survey, Auckland Islands December 2015 – February 2016 Graham C. Parker, Chris G. Muller and Kalinka Rexer‐Huber Department of Conservation, Conservation Services Programme, Contract 4655‐4 1 Northern giant petrel Macronectes halli breeding population survey, Auckland Islands December 2015– February 2016 Department of Conservation, Conservation Services Programme, Contract 4655‐4 Graham C. Parker1*, Chris G. Muller3 & Kalinka Rexer‐Huber1,2 1 Parker Conservation, 126 Maryhill Tce, Dunedin New Zealand 2 University of Otago, PO Box 56, Dunedin New Zealand 3Massey University Manawatu, Private Bag 11 222, Palmerston North 4442, New Zealand *Corresponding author: [email protected] Please cite as: Parker, G.C., Muller, C.G., Rexer‐Huber, K. 2016. Northern giant petrel Macronectes halli breeding population survey, Auckland Islands, December 2015 – February 2016. Report to the Conservation Services Programme, Department of Conservation. Parker Conservation, Dunedin 2 Executive summary Northern giant petrels Macronectes halli are a large, southern hemisphere fulmarine petrel that face conservation threats both in the terrestrial and marine environment. Introduced mammalian predators at breeding sites cause nesting failures and in some instances may also depredate adults. In the marine environment Northern giant petrels are threatened by capture in longline and trawl fisheries, oil pollution, shooting by fishers for bait stealing and the effects of climate change. The contemporary size and the population trends of Northern giant petrels on New Zealand islands are not known. Records of their numbers in the Auckland Islands are based solely on anecdotal evidence, and the most recent summary dates to the 1980s. We estimated the size of the Northern giant petrel breeding population and describe their spatial distribution in the Auckland Islands. -

Managementframeworksf
Papers and Proceedings ofthe Royal Society of Tasmania, Volume 141(1), 2007 29 MANAGEMENTFRAMEWORKSFOR THE NEWZEALAND SUB-ANTARCTIC ISLANDS by A. D. Roberts Roberts, A.D. 2007 (23:xi): Management frameworks for the New Zealand sub-Antarctic islands. Papers and Proceedings of the Royal Society of Tasmania 141(1): 29-32. https://doi.org/10.26749/rstpp.141.1.29 ISSN 0080-4703. Department of Conservation, PO Box 743, Invercargill, New Zealand. Email: [email protected] Thefive island groups known as the New Zealand sub-Antarctic islands - Antipodes Islands, Auckland Islands, Bounty Islands, Campbell Island and Snares Islands - are perhaps better regarded as cool temperate although they share many features and many management issues with islands south of the Antarctic Convergence. The Snares and Bounty islands have granitic substrates; the Antipodes, Auckland and Campbell islands are of volcanic origin. Marine mammals and seabirds are important components of the fauna. Terrestrial Hora and inver tebrate fauna show high levels of endemism. Several species of alien vertebrates have been successfully eradicated. The islands are managed by the New Zealand Department of Conservation as National Nature Reserves, and together are recognised as a World Heritage Site. Key Words: sub-Antarctic islands, cool temperate, Antipodes Islands, Auckland Islands, Bounty Islands, Campbell Island, Snares Islands, World Heritage Site, alien species removal. TheNew Zealand sub-Antarctic islands are located south of There is evidence of Polynesian v1s1tation in the pre New Zealand in the Pacific sector of the Southern Ocean. European period (Peat 2003). European discovery was in Although these islands are not truly sub-Antarctic but more 1791. -
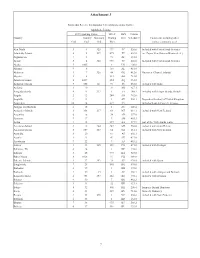
Attachment 3
Attachment 3 Points that Receive International Telecommunications Traffic: Alphabetic Listing FCC reporting Codes ITU-T FIPS Census Country Country Summary Dialing 10-3 Schedule C Comments, including other Code Code Code Plan names commonly used Abu Dhabi 3 1 325 971 TC 520.0 Included with United Arab Emirates Admiralty Islands 8 2 237 675 PP 604.0 inc. Papua New Guinea (Bismarck A.) Afghanistan 7 3 93 AF 531.0 Ajman 3 4 325 971 TC 520.0 Included with United Arab Emirates Alaska 5 1005 1 US 100.0 Albania 9 6 355 AL 481.0 Alderney 1 7 326 44 GK 412.0 Guernsey (Channel Islands) Algeria 2 8 213 AG 721.0 American Samoa 8 1009 684 AQ 951.0 Andaman Islands 7 355 141 91 IN 533.0 included with India Andorra 1 10 33 AN 427.1 Anegada Islands 4 11 337 1 VI 248.2 included with Virgin Islands, British Angola 2 12 244 AO 762.0 Anguilla 4 13 1 AV 248.1 Dependent territory of United Kingdom Antarctica 10 14 672 AY Includes Scott & Casey U.S. bases Antigua and Barbuda 4 15 1 AC 248.4 Antipodes Islands 8 356 217 64 NZ 614.1 included with New Zealand Argentina 6 16 54 AR 357.0 Armenia 9 17 7 AM 463.1 Aruba 4 18 297 AA 277.9 part of the Netherlands realm Ascension Island 2 19 264 247 SH 758.0 included with Saint Helena Auckland Islands 8 357 217 64 NZ 614.1 included with New Zealand Australia 8 20 61 AS 602.1 Austria 1 21 43 AU 433.0 Azerbaijan 9 22 7 AJ 463.2 Azores 1 23 245 351 PO 471.0 included with Portugal Bahamas, The 4 24 1 BF 236.0 Bahrain 3 25 973 BA 525.0 Baker Island 8 1026 11 FQ 980.0 Balearic Islands 1 27 293 34 SP 470.0 included with Spain -

New Zealand Sub-Antarctic Islands New Zealand
NEW ZEALAND SUB-ANTARCTIC ISLANDS NEW ZEALAND The New Zealand Sub-Antarctic Islands consist of five remote and windswept island groups in the Southern Ocean south and south-east of New Zealand. The islands, lying between the Antarctic and Subtropical Convergences, are oases of high productivity, biodiversity, dense populations and endemism for birds, ocean life, plants and invertebrates. Of the 126 species of birds, 40 are seabirds of which 5 breed nowhere else in the world. They have among the most southerly forests in the world, an unusual flora of megaherbs and some small islands never colonised by man. COUNTRY New Zealand NAME New Zealand Sub-Antarctic Islands NATURAL WORLD HERITAGE SERIAL SITE 1998: Inscribed on the World Heritage List under Natural Criteria ix and x. STATEMENT OF OUTSTANDING UNIVERSAL VALUE [pending] The UNESCO World Heritage Committee issued the following statement at the time of inscription: Justification for Inscription Criterion (ix): The New Zealand Sub-Antarctic Islands display a pattern of immigration of species, diversifications and emergent endemism, offering particularly good opportunities for research into the dynamics of island ecology. Criterion (x): The New Zealand Sub-Antarctic Islands are remarkable for their high level of biodiversity, population densities,and for endemism in birds, plants and invertebrates. The bird and plant life, especially the endemic albatrosses, cormorants, landbirds and “megaherbs” are unique to the islands. IUCN MANAGEMENT CATEGORY Auckland Islands National Nature Reserve -

The Following Is a Section of a Document Properly Cited As: Snyder, N., Mcgowan, P., Gilardi, J., and Grajal, A. (Eds.) (2000) P
The following is a section of a document properly cited as: Snyder, N., McGowan, P., Gilardi, J., and Grajal, A. (eds.) (2000) Parrots. Status Survey and Conservation Action Plan 2000–2004. IUCN, Gland, Switzerland and Cambridge, UK. x + 180 pp. © 2000 International Union for Conservation of Nature and Natural Resources and the World Parrot Trust It has been reformatted for ease of use on the internet . The resolution of the photographs is considerably reduced from the printed version. If you wish to purchase a printed version of the full document, please contact: IUCN Publications Unit 219c Huntingdon Road, Cambridge, CB3 0DL, UK. Tel: (44) 1223 277894 Fax: (44) 1223 277175 Email: [email protected] The World Parrot Trust World Parrot Trust UK World Parrot Trust USA Order on-line at: Glanmor House PO Box 353 www.worldparrottrust.org Hayle, Cornwall Stillwater, MN 55082 TR27 4HB, United Kingdom Tel: 651 275 1877 Tel: (44) 1736 753365 Fax: 651 275 1891 Fax (44) 1736 751028 Island Press Box 7, Covelo, California 95428, USA Tel: 800 828 1302, 707 983 6432 Fax: 707 983 6414 E-mail: [email protected] Order on line: www.islandpress.org The views expressed in this publication are those of the authors and do not necessarily reflect those of IUCN or the Species Survival Commission. Published by: IUCN, Gland, Switzerland, and Cambridge, UK. Copyright: © 2000 International Union for Conservation of Nature and Natural Resources and the World Parrot Trust Reproduction of this publication for educational and other non-commercial purposes is authorised without prior written permission from the copyright holders provided the source is fully acknowledged. -

Adobe PDF, Job 6
Noms français des oiseaux du Monde par la Commission internationale des noms français des oiseaux (CINFO) composée de Pierre DEVILLERS, Henri OUELLET, Édouard BENITO-ESPINAL, Roseline BEUDELS, Roger CRUON, Normand DAVID, Christian ÉRARD, Michel GOSSELIN, Gilles SEUTIN Éd. MultiMondes Inc., Sainte-Foy, Québec & Éd. Chabaud, Bayonne, France, 1993, 1re éd. ISBN 2-87749035-1 & avec le concours de Stéphane POPINET pour les noms anglais, d'après Distribution and Taxonomy of Birds of the World par C. G. SIBLEY & B. L. MONROE Yale University Press, New Haven and London, 1990 ISBN 2-87749035-1 Source : http://perso.club-internet.fr/alfosse/cinfo.htm Nouvelle adresse : http://listoiseauxmonde.multimania.