Nguyen, Vuvi H., a Cadaveric Investigation of the Dorsal Scapular Nerve
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Risk of Encountering Dorsal Scapular and Long Thoracic Nerves During Ultrasound-Guided Interscalene Brachial Plexus Block with Nerve Stimulator
Korean J Pain 2016 July; Vol. 29, No. 3: 179-184 pISSN 2005-9159 eISSN 2093-0569 http://dx.doi.org/10.3344/kjp.2016.29.3.179 | Original Article | Risk of Encountering Dorsal Scapular and Long Thoracic Nerves during Ultrasound-guided Interscalene Brachial Plexus Block with Nerve Stimulator Department of Anesthesiology and Pain Medicine, Wonkwang University College of Medicine, Wonkwang Institute of Science, Iksan, *Department of Anesthesiology and Pain Medicine, Presbyterian Medical Center, University of Seonam College of Medicine, Jeonju, Korea Yeon Dong Kim, Jae Yong Yu*, Junho Shim*, Hyun Joo Heo*, and Hyungtae Kim* Background: Recently, ultrasound has been commonly used. Ultrasound-guided interscalene brachial plexus block (IBPB) by posterior approach is more commonly used because anterior approach has been reported to have the risk of phrenic nerve injury. However, posterior approach also has the risk of causing nerve injury because there are risks of encountering dorsal scapular nerve (DSN) and long thoracic nerve (LTN). Therefore, the aim of this study was to evaluate the risk of encountering DSN and LTN during ultrasound-guided IBPB by posterior approach. Methods: A total of 70 patients who were scheduled for shoulder surgery were enrolled in this study. After deciding insertion site with ultrasound, awake ultrasound-guided IBPB with nerve stimulator by posterior approach was performed. Incidence of muscle twitches (rhomboids, levator scapulae, and serratus anterior muscles) and current intensity immediately before muscle twitches disappeared were recorded. Results: Of the total 70 cases, DSN was encountered in 44 cases (62.8%) and LTN was encountered in 15 cases (21.4%). Both nerves were encountered in 10 cases (14.3%). -
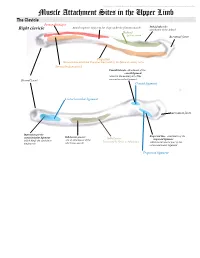
Muscle Attachment Sites in the Upper Limb
This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. Muscle Attachment Sites in the Upper Limb The Clavicle Pectoralis major Smooth superior surface of the shaft, under the platysma muscle Deltoid tubercle: Right clavicle attachment of the deltoid Deltoid Axillary nerve Acromial facet Trapezius Sternocleidomastoid and Trapezius innervated by the Spinal Accessory nerve Sternocleidomastoid Conoid tubercle, attachment of the conoid ligament which is the medial part of the Sternal facet coracoclavicular ligament Conoid ligament Costoclavicular ligament Acromial facet Impression for the Trapezoid line, attachment of the costoclavicular ligament Subclavian groove: Subclavius trapezoid ligament which binds the clavicle to site of attachment of the Innervated by Nerve to Subclavius which is the lateral part of the the first rib subclavius muscle coracoclavicular ligament Trapezoid ligament This document was created by Alex Yartsev ([email protected]); if I have used your data or images and forgot to reference you, please email me. The Scapula Trapezius Right scapula: posterior Levator scapulae Supraspinatus Deltoid Deltoid and Teres Minor are innervated by the Axillary nerve Rhomboid minor Levator Scapulae, Rhomboid minor and Rhomboid Major are innervated by the Dorsal Scapular Nerve Supraspinatus and Infraspinatus innervated by the Suprascapular nerve Infraspinatus Long head of triceps Rhomboid major Teres Minor Teres Major Teres Major -

Examination of the Shoulder Bruce S
Examination of the Shoulder Bruce S. Wolock, MD Towson Orthopaedic Associates 3 Joints, 1 Articulation 1. Sternoclavicular 2. Acromioclavicular 3. Glenohumeral 4. Scapulothoracic AC Separation Bony Landmarks 1. Suprasternal notch 2. Sternoclavicular joint 3. Coracoid 4. Acromioclavicular joint 5. Acromion 6. Greater tuberosity of the humerus 7. Bicipital groove 8. Scapular spine 9. Scapular borders-vertebral and lateral Sternoclavicular Dislocation Soft Tissues 1. Rotator Cuff 2. Subacromial bursa 3. Axilla 4. Muscles: a. Sternocleidomastoid b. Pectoralis major c. Biceps d. Deltoid Congenital Absence of Pectoralis Major Pectoralis Major Rupture Soft Tissues (con’t) e. Trapezius f. Rhomboid major and minor g. Latissimus dorsi h. Serratus anterior Range of Motion: Active and Passive 1. Abduction - 90 degrees 2. Adduction - 45 degrees 3. Extension - 45 degrees 4. Flexion - 180 degrees 5. Internal rotation – 90 degrees 6. External rotation – 45 degrees Muscle Testing 1. Flexion a. Primary - Anterior deltoid (axillary nerve, C5) - Coracobrachialis (musculocutaneous nerve, C5/6 b. Secondary - Pectoralis major - Biceps Biceps Rupture- Longhead Muscle Testing 2. Extension a. Primary - Latissimus dorsi (thoracodorsal nerve, C6/8) - Teres major (lower subscapular nerve, C5/6) - Posterior deltoid (axillary nerve, C5/6) b. Secondary - Teres minor - Triceps Abduction Primary a. Middle deltoid (axillary nerve, C5/6) b. Supraspinatus (suprascapular nerve, C5/6) Secondary a. Anterior and posterior deltoid b. Serratus anterior Deltoid Ruputure Axillary Nerve Palsy Adduction Primary a. Pectoralis major (medial and lateral pectoral nerves, C5-T1 b. Latissimus dorsi (thoracodorsal nerve, C6/8) Secondary a. Teres major b. Anterior deltoid External Rotation Primary a. Infraspinatus (suprascapular nerve, C5/6) b. Teres minor (axillary nerve, C5) Secondary a. -

Dorsal Scapular Nerve Neuropathy: a Narrative Review of the Literature Brad Muir, Bsc.(Hons), DC, FRCCSS(C)1
ISSN 0008-3194 (p)/ISSN 1715-6181 (e)/2017/128–144/$2.00/©JCCA 2017 Dorsal scapular nerve neuropathy: a narrative review of the literature Brad Muir, BSc.(Hons), DC, FRCCSS(C)1 Objective: The purpose of this paper is to elucidate Objectif : Ce document a pour objectif d’élucider this little known cause of upper back pain through a cette cause peu connue de douleur dans le haut du narrative review of the literature and to discuss the dos par un examen narratif de la littérature, ainsi que possible role of the dorsal scapular nerve (DSN) in de discuter du rôle possible du nerf scapulaire dorsal the etiopathology of other similar diagnoses in this (NSD) dans l’étiopathologie d’autres diagnostics area including cervicogenic dorsalgia (CD), notalgia semblables dans ce domaine, y compris la dorsalgie paresthetica (NP), SICK scapula and a posterolateral cervicogénique (DC), la notalgie paresthésique (NP), arm pain pattern. l’omoplate SICK et un schéma de douleur postéro- Background: Dorsal scapular nerve (DSN) latérale au bras. neuropathy has been a rarely thought of differential Contexte : La neuropathie du nerf scapulaire dorsal diagnosis for mid scapular, upper to mid back and (NSD) constitue un diagnostic différentiel rare pour la costovertebral pain. These are common conditions douleur mi-scapulaire, costo-vertébrale et au bas/haut presenting to chiropractic, physiotherapy, massage du dos. Il s’agit de troubles communs qui surgissent therapy and medical offices. dans les cabinets de chiropratique, de physiothérapie, de Methods: The methods used to gather articles for this massothérapie et de médecin. paper included: searching electronic databases; and Méthodologie : Les méthodes utilisées pour hand searching relevant references from journal articles rassembler les articles de ce document comprenaient la and textbook chapters. -

Electrodiagnosis of Brachial Plexopathies and Proximal Upper Extremity Neuropathies
Electrodiagnosis of Brachial Plexopathies and Proximal Upper Extremity Neuropathies Zachary Simmons, MD* KEYWORDS Brachial plexus Brachial plexopathy Axillary nerve Musculocutaneous nerve Suprascapular nerve Nerve conduction studies Electromyography KEY POINTS The brachial plexus provides all motor and sensory innervation of the upper extremity. The plexus is usually derived from the C5 through T1 anterior primary rami, which divide in various ways to form the upper, middle, and lower trunks; the lateral, posterior, and medial cords; and multiple terminal branches. Traction is the most common cause of brachial plexopathy, although compression, lacer- ations, ischemia, neoplasms, radiation, thoracic outlet syndrome, and neuralgic amyotro- phy may all produce brachial plexus lesions. Upper extremity mononeuropathies affecting the musculocutaneous, axillary, and supra- scapular motor nerves and the medial and lateral antebrachial cutaneous sensory nerves often occur in the context of more widespread brachial plexus damage, often from trauma or neuralgic amyotrophy but may occur in isolation. Extensive electrodiagnostic testing often is needed to properly localize lesions of the brachial plexus, frequently requiring testing of sensory nerves, which are not commonly used in the assessment of other types of lesions. INTRODUCTION Few anatomic structures are as daunting to medical students, residents, and prac- ticing physicians as the brachial plexus. Yet, detailed understanding of brachial plexus anatomy is central to electrodiagnosis because of the plexus’ role in supplying all motor and sensory innervation of the upper extremity and shoulder girdle. There also are several proximal upper extremity nerves, derived from the brachial plexus, Conflicts of Interest: None. Neuromuscular Program and ALS Center, Penn State Hershey Medical Center, Penn State College of Medicine, PA, USA * Department of Neurology, Penn State Hershey Medical Center, EC 037 30 Hope Drive, PO Box 859, Hershey, PA 17033. -

A Cadaveric Investigation of the Dorsal Scapular Nerve
Hindawi Publishing Corporation Anatomy Research International Volume 2016, Article ID 4106981, 5 pages http://dx.doi.org/10.1155/2016/4106981 Research Article A Cadaveric Investigation of the Dorsal Scapular Nerve Vuvi H. Nguyen,1 Hao (Howe) Liu,2 Armando Rosales,1 and Rustin Reeves1 1 Center for Anatomical Sciences, University of North Texas Health Science Center, Fort Worth, TX 76107, USA 2Department of Physical Therapy, University of North Texas Health Science Center, Fort Worth, TX 76107, USA Correspondence should be addressed to Rustin Reeves; [email protected] Received 24 June 2016; Accepted 19 July 2016 Academic Editor: Udo Schumacher Copyright © 2016 Vuvi H. Nguyen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Compression of the dorsal scapular nerve (DSN) is associated with pain in the upper extremity and back. Even though entrapment of the DSN within the middle scalene muscle is typically the primary cause of pain, it is still easily missed during diagnosis. The purpose of this study was to document the DSN’s anatomy and measure the oblique course it takes with regard to the middle scalene muscle. From 20 embalmed adult cadavers, 23 DSNs were documented regarding the nerve’s spinal root origin, anatomical route, and muscular innervations. A transverse plane through the laryngeal prominence was established to measure the distance of the DSN from this plane as it enters, crosses, and exits the middle scalene muscle. Approximately 70% of the DSNs originated from C5, with 74% piercing the middle scalene muscle. -
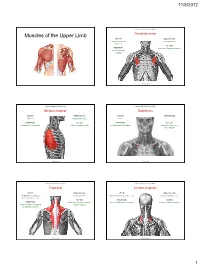
Muscles of the Upper Limb.Pdf
11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles of the Upper Limb Pectoralis minor ORIGIN: INNERVATION: anterior surface of pectoral nerves ribs 3 – 5 ACTION: INSERTION: protracts / depresses scapula coracoid process (scapula) (Anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Serratus anterior Subclavius ORIGIN: INNERVATION: ORIGIN: INNERVATION: ribs 1 - 8 long thoracic nerve rib 1 ---------------- INSERTION: ACTION: INSERTION: ACTION: medial border of scapula rotates scapula laterally inferior surface of scapula stabilizes / depresses pectoral girdle (Lateral view) (anterior view) Muscles Stabilizing Pectoral Girdle Muscles Stabilizing Pectoral Girdle Trapezius Levator scapulae ORIGIN: INNERVATION: ORIGIN: INNERVATION: occipital bone / spinous accessory nerve transverse processes of C1 – C4 dorsal scapular nerve processes of C7 – T12 ACTION: INSERTION: ACTION: INSERTION: stabilizes / elevates / retracts / upper medial border of scapula elevates / adducts scapula acromion / spine of scapula; rotates scapula lateral third of clavicle (Posterior view) (Posterior view) 1 11/8/2012 Muscles Stabilizing Pectoral Girdle Muscles Moving Arm Rhomboids Pectoralis major (major / minor) ORIGIN: INNERVATION: ORIGIN: INNERVATION: spinous processes of C7 – T5 dorsal scapular nerve sternum / clavicle / ribs 1 – 6 dorsal scapular nerve INSERTION: ACTION: INSERTION: ACTION: medial border of scapula adducts / rotates scapula intertubucular sulcus / greater tubercle flexes / medially rotates / (humerus) adducts -

5223.0400 Peripheral Nervous System; Upper Extremity-Motor Loss
1 REVISOR 5223.0400 5223.0400 PERIPHERAL NERVOUS SYSTEM; UPPER EXTREMITY-MOTOR LOSS. Subpart 1. General. For permanent partial impairment to the peripheral nerves, plexuses, and nerve roots of the upper extremity resulting from nerve injury or disease, and if there is total loss of motor function for those particular portions of the body served by the peripheral nerve, plexus, or nerve root, disability to the whole body is as provided in subparts 2 to 6. A. Total or complete motor loss means that motor function is less than muscle strength grade 2/5. B. If injury to a nerve, plexus, or nerve root results only in sensory loss, the rating is as provided in part 5223.0410. C. If motor loss occurs together with sensory loss, the rating under this part may be combined as described in part 5223.0300, subpart 3, item E, with the rating under part 5223.0410. D. The ratings in this part include the rating of the impairment due to any restriction of range of motion or ankylosis at any joint of the affected member that is strictly the result of the nerve lesion and no further rating for those losses shall be combined with ratings under this part. Subp. 2. Peripheral nerve. There is total or complete motor loss of the peripheral nerve, and signs or symptoms of organic disease or injury are present, and there is anatomic loss or alteration: A. median nerve: (1) entire motor distribution involved, 33 percent; (2) involving the flexor pollicis longus, flexor digitorum profundus (index), flexor digitorum superficialis, pronator quadratus, and intrinsic muscles of the hand, 21 percent; (3) involving the flexor pollicis longus, flexor digitorum profundus (index), and pronator quadratus (anterior interosseous syndrome), 15 percent; B. -

Upper Extremity Compression Neuropathies
Peripheral Nerve Injury in the Athlete: Upper and Lower Limb Entrapment Neuropathies Greg Moore, MD Sports and Spine Rehabilitation NeuroSpine Institute Outline Review common nerve entrapment and injury syndromes, particularly related to sports Review pertinent anatomy to each nerve Review typical symptoms Discuss pathophysiology Discuss pertinent diagnostic tests and treatment options Neuropathy Mononeuropathies Median Femoral Pronator Teres Intrapelvic Anterior Interosseous Nerve Inguinal Ligament Carpal Tunnel Sciatic Ulnar Piriformis Cubital Tunnel Peroneal Guyon’s Canal Fibular Head Radial Axilla Tibial Spiral Groove Tarsal Tunnel Posterior Interosseous Nerve Sports Medicine Pearls Utilize your athletic trainers Individualize your diagnostic and treatment approach based on multiple factors Age Sport Level of Sport (HS, college, professional) Position Sports Medicine Pearls Time in the season Degree of pain/disability Desire of the patient/parents Coach’s desires/level of concern Cost (rarely discuss with the coach) Danger of a delay in diagnosis Impact to the team Obtaining the History Pain questions- location, duration, type, etc. Presence and location of numbness and paresthesias Exertional fatigue and/or weakness Subjective muscle atrophy Symptom onset- insidious or post-traumatic Exacerbating activities History (continued) Changes in exercise duration, intensity or frequency New techniques or equipment Past medical history and review of systems Diabetes Hypercoaguable state Depression/anxiety -

Neuromuscular Variations in the Posterior Triangle of the Neck – a Case Report
eISSN 1303-1775 • pISSN 1303-1783 Neuroanatomy (2008) 7: 8–9 Case Report Neuromuscular variations in the posterior triangle of the neck – a case report Published online 11 March, 2008 © http://www.neuroanatomy.org Bincy M. GEORGE ABSTRACT Satheesha B. NAYAK The lower part of the posterior triangle of the neck is one of the important areas of the body because of the presence of brachial plexus. The thorough knowledge of anatomy and anatomical variations in this region are important for surgeons, anesthesiologist and physiotherapists. We report here the neuromuscular variations at the lower part of the posterior triangle of the neck. In the current case, the dorsal scapular nerve made a Melaka Manipal Medical College (Manipal Campus), International Centre for Health loop around the deep branch of transverse cervical artery. There was an additional muscle arising from the Sciences, Madhav Nagar, Manipal, Udupi District, Karnataka State, INDIA. first rib and getting inserted to the inner surface of the superior angle of the scapula. The long thoracic nerve pierced this additional muscle. The loop of dorsal scapular nerve around the artery may lead to neurovascular symptoms and the abnormal muscle pierced by the long thoracic nerve may cause to neuromuscular symptoms. © Neuroanatomy. 2008; 7: 8–9. Bincy M. George Lecturer of Anatomy Melaka Manipal Medical College (Manipal Campus) International Centre for Health Sciences Madhav Nagar, Manipal Udupi District, Karnataka State, 576 104 INDIA. +91 820 2922519 +91 820 2571905 [email protected] Received 14 August 2007; accepted 12 February 2008 Key words [variations] [dorsal scapular nerve] [long thoracic nerve] Introduction originated from the upper surface of the first rib near the Dorsal scapular nerve arises from the ventral ramus attachment of scalenus medius and inserted into the inner of the fifth cervical spinal nerve. -

Muscles of the Upper Extremity
MUSCLES OF THE UPPER EXTREMITY: Movement of the shoulder and arm: Anterior Axioappendicular Muscles: Pectoralis major O: (clavicular head) medial 1/2 of Clavicle, All fibers : Adducts and medially rotates humerus at Nerves: Lateral and medial pectoral nerves (Sternocostal) Sternum, Anterior suface of shoulder. Also, draws scapula anteriorly and inferiorly Roots: Clavicular (C 5-6), Sternocostal (C 7-8, T1) Ribs 1-6, aponeurosis of External Oblique Clavicular and Sterno fibers : flexes and I: Lateral lip of intertubercular sulcus horizonly adducts humerus. of humerus. Costal fibers : extends humerus. S: Adduction: Latissimus Dorsi, Teres (major & minor), Extension: Posterior deltoid, Latissimus dorsi, Medial rotation: Latissimus Dorsi, Anterior Deltoid, Infraspinatus, Long head Triceps, coracobrachialis teres major, Long head Tricep Teres major, subscapularis A: Abduction: All 3 parts of Deltoid, Supraspinatus, Flexion: Anterior Deltoid, Biceps brachii Lateral rotation: Infraspinatus, Teres minor, coracobrachialis Posterior Deltoid Pectoralis minor O: Anterior superior surface of ribs 3-5 With ribs fixed: Nerve: Medial pectoral nerves sometimes rib 6 Depresses, abducts, downwardly rotate scapula. Roots: (C8 and T1) I: Coracoid process of scapula. With scapula fixed: Elevates 3rd through 5th ribs during forced inspiration. S: Abduction: Serratus Anterior Depression: Lower Trapezius, Serratus anterior Downward rotation: Rhomboid major and minor, Levator scapula, A: Adduction: Romboideus Major and Minor, middle Tapezius Elevation : Upper Trapezius, -

Research Article a Cadaveric Investigation of the Dorsal Scapular Nerve
Hindawi Publishing Corporation Anatomy Research International Volume 2016, Article ID 4106981, 5 pages http://dx.doi.org/10.1155/2016/4106981 Research Article A Cadaveric Investigation of the Dorsal Scapular Nerve Vuvi H. Nguyen,1 Hao (Howe) Liu,2 Armando Rosales,1 and Rustin Reeves1 1 Center for Anatomical Sciences, University of North Texas Health Science Center, Fort Worth, TX 76107, USA 2Department of Physical Therapy, University of North Texas Health Science Center, Fort Worth, TX 76107, USA Correspondence should be addressed to Rustin Reeves; [email protected] Received 24 June 2016; Accepted 19 July 2016 Academic Editor: Udo Schumacher Copyright © 2016 Vuvi H. Nguyen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Compression of the dorsal scapular nerve (DSN) is associated with pain in the upper extremity and back. Even though entrapment of the DSN within the middle scalene muscle is typically the primary cause of pain, it is still easily missed during diagnosis. The purpose of this study was to document the DSN’s anatomy and measure the oblique course it takes with regard to the middle scalene muscle. From 20 embalmed adult cadavers, 23 DSNs were documented regarding the nerve’s spinal root origin, anatomical route, and muscular innervations. A transverse plane through the laryngeal prominence was established to measure the distance of the DSN from this plane as it enters, crosses, and exits the middle scalene muscle. Approximately 70% of the DSNs originated from C5, with 74% piercing the middle scalene muscle.