Nesting Ecology and Habitat Use of Chelydra Serpentina in an Area Modified by Agricultural and Industrial Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nest Check Data Sheet
Nest check data sheet Use this form to describe your nest site and to record data from each visit. Use a separate form for each nest monitored and each new nesting attempt. See back for explanations of codes and fields. When finished, please enter completed forms online at: www.nestwatch.org. Year _________________________ Species ______________________________________________________________________________ 1. NEST SITE LOCATION 2. DESCRIPTION (see key on back) Nest site name Nest is located (circle one) IN ON UNDER __________________________________________________ Nesting substrate _______________________________ Cavity orientation (circle one) N, S, E, W, NE, SE, NW, SW Address: Nearest street address OR Cavity opening width __________________ ❏ in. or ❏ cm __________________________________________________ Predator guard ❏ None or ❏ Type: __________________ __________________________________________________ Habitat within 1 arm length _________________________ Latitude (decimal degrees; ex 47.67932) Human modified description ___________________ N _______________________________________________ Habitat within 1 football field length _________________ Longitude (decimal degrees; ex -76.45448) Human modified description ___________________ W _______________________________________________ Height above ground ____________________❏ ft. or ❏ m 3. BREEDING DATA If eggs or young are present but not countable, enter “u” for unknown. DATE HOST SPECIES STATUS & ACTIVITY CODES COWBIRD ACTIVITY MORE INFO Month / Day Live Dead Nest Adult Young -

A Molecular Phylogeny for Marine Turtles: Trait Mapping, Rate
Proc. Natl. Acad. Sci. USA Vol. 90, pp. 5574-5577, June 1993 Evolution A molecular phylogeny for marine turtles: Trait mapping, rate assessment, and conservation relevance (conservation genetlcs/cytochrome b/molecular systematics/mitochondrial DNA) BRIAN W. BOWEN*, WILLIAM S. NELSON, AND JOHN C. AVISE Department of Genetics, University of Georgia, Athens, GA 30602 Contributed by John C. Avise, March 25, 1993 ABSTRACT Nucleotide sequences from the cytochrome b Table 1. A recent taxonomy for marine turtles, with problematic gene of mitochondrial DNA were employed to resolve phylo- areas indicated by parenthetical questions genetic controversies and to assess molecular evolutionary rates Order Testudines-all extant turtles, freshwater, terrestrial, and in marine turtles (Chelonioidea). Findings of special relevance marine to conservation biology include discovery of a distant relation- Family Dermochelyidae-Dermochelys coriacea (leatherback) ship betweenNatatorand other chelonild species, the paraphyly (Are marine turtles monophyletic? Where does this species lie ofChelonia mydas with respect to Chelonia agassizi, and genetic with regard to broader turtle phylogeny?) distinctiveness of Lepidochelys kempi from Lepidochelys oliva- Family Cheloniidae cea. A longstanding debate in evolutionary ecology was re- Tribe Chelonini solved by phylogenetic mapping of dietary habits, which indi- Genus Chelonia-C. mydas (green) cates that the spongivore Ereinochelys imbricata evolved from C. agassizi (black) a carnivorous rather than a herbivorous ancestor. Sequence (Are these two forms distinct species?) divergences at intergeneric and interfamilial levels, when as- Genus Natator-N. depressus (flatback) sessed against fossil-based separation times, support previous (Is this species a close ally of the green turtle, or perhaps suggestions (from microevolutionary comparisons) that mito- allied more closely to members of the Carettini?) chondrial DNA in marine turtles evolves much more slowly Tribe Carettini than under the "conventional" vertebrate clock. -
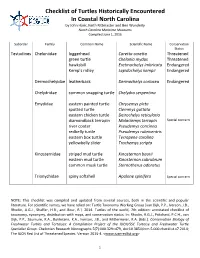
N.C. Turtles Checklist
Checklist of Turtles Historically Encountered In Coastal North Carolina by John Hairr, Keith Rittmaster and Ben Wunderly North Carolina Maritime Museums Compiled June 1, 2016 Suborder Family Common Name Scientific Name Conservation Status Testudines Cheloniidae loggerhead Caretta caretta Threatened green turtle Chelonia mydas Threatened hawksbill Eretmochelys imbricata Endangered Kemp’s ridley Lepidochelys kempii Endangered Dermochelyidae leatherback Dermochelys coriacea Endangered Chelydridae common snapping turtle Chelydra serpentina Emydidae eastern painted turtle Chrysemys picta spotted turtle Clemmys guttata eastern chicken turtle Deirochelys reticularia diamondback terrapin Malaclemys terrapin Special concern river cooter Pseudemys concinna redbelly turtle Pseudemys rubriventris eastern box turtle Terrapene carolina yellowbelly slider Trachemys scripta Kinosternidae striped mud turtle Kinosternon baurii eastern mud turtle Kinosternon subrubrum common musk turtle Sternotherus odoratus Trionychidae spiny softshell Apalone spinifera Special concern NOTE: This checklist was compiled and updated from several sources, both in the scientific and popular literature. For scientific names, we have relied on: Turtle Taxonomy Working Group [van Dijk, P.P., Iverson, J.B., Rhodin, A.G.J., Shaffer, H.B., and Bour, R.]. 2014. Turtles of the world, 7th edition: annotated checklist of taxonomy, synonymy, distribution with maps, and conservation status. In: Rhodin, A.G.J., Pritchard, P.C.H., van Dijk, P.P., Saumure, R.A., Buhlmann, K.A., Iverson, J.B., and Mittermeier, R.A. (Eds.). Conservation Biology of Freshwater Turtles and Tortoises: A Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Research Monographs 5(7):000.329–479, doi:10.3854/crm.5.000.checklist.v7.2014; The IUCN Red List of Threatened Species. -

Gopher Tortoise Demographic Responses to a Novel Disturbance Regime
The Journal of Wildlife Management 1–10; 2019; DOI: 10.1002/jwmg.21774 Research Article Gopher Tortoise Demographic Responses to a Novel Disturbance Regime HUNTER J. HOWELL ,1,2 Department of Biology, University of Miami, 1301 Memorial Drive, Coral Gables, FL 33146, USA BETSIE B. ROTHERMEL,2 Archbold Biological Station, 123 Main Drive, Venus, FL 33960, USA K. NICOLE WHITE, Daniel B. Warnell School of Forestry and Natural Resources, University of Georgia, 180 E Green Street, Athens, GA 30602, USA CHRISTOPHER A. SEARCY, Department of Biology, University of Miami, 1301 Memorial Drive, Coral Gables, FL 33146, USA ABSTRACT The long‐term viability of gopher tortoise (Gopherus polyphemus) populations is jeopardized by increased urbanization and habitat degradation owing to fire suppression. Because the species’ remaining natural habitats in the southeastern United States exist within a mosaic of anthropogenic land uses, it is important to understand demographic responses to contrasting land uses and habitat management regimes. We examined differences in demographic parameters among fire‐suppressed sandhill, restored sandhill, and former sandhill (i.e., ruderal) land use‐land cover (LULC) types at Archbold Biological Station in south‐ central Florida, USA. Using Program MARK, we estimated population size, and sex‐specific and LULC‐specific survivorship based on 6 years of mark‐recapture data. We also analyzed individual growth trajectories and clutch sizes to determine whether growth rates or reproductive output differed among LULC types. Tortoises in an open, ruderal field occurred at a higher density (7.79/ha) than in adjacent restored (1.43/ha) or fire‐suppressed (0.40/ha) sandhill. Despite this higher density, both adult survivorship and body size were significantly higher in the ruderal field. -

The Conservation Biology of Tortoises
The Conservation Biology of Tortoises Edited by Ian R. Swingland and Michael W. Klemens IUCN/SSC Tortoise and Freshwater Turtle Specialist Group and The Durrell Institute of Conservation and Ecology Occasional Papers of the IUCN Species Survival Commission (SSC) No. 5 IUCN—The World Conservation Union IUCN Species Survival Commission Role of the SSC 3. To cooperate with the World Conservation Monitoring Centre (WCMC) The Species Survival Commission (SSC) is IUCN's primary source of the in developing and evaluating a data base on the status of and trade in wild scientific and technical information required for the maintenance of biological flora and fauna, and to provide policy guidance to WCMC. diversity through the conservation of endangered and vulnerable species of 4. To provide advice, information, and expertise to the Secretariat of the fauna and flora, whilst recommending and promoting measures for their con- Convention on International Trade in Endangered Species of Wild Fauna servation, and for the management of other species of conservation concern. and Flora (CITES) and other international agreements affecting conser- Its objective is to mobilize action to prevent the extinction of species, sub- vation of species or biological diversity. species, and discrete populations of fauna and flora, thereby not only maintain- 5. To carry out specific tasks on behalf of the Union, including: ing biological diversity but improving the status of endangered and vulnerable species. • coordination of a programme of activities for the conservation of biological diversity within the framework of the IUCN Conserva- tion Programme. Objectives of the SSC • promotion of the maintenance of biological diversity by monitor- 1. -

Relative Abundance, Population Structure, and Habitat
University of Missouri, St. Louis IRL @ UMSL Theses Graduate Works 11-18-2010 Relative Abundance, Population Structure, and Habitat Utilization of the Alligator Snapping Turtle (Macrochelys temminckii) and Eastern Snapping Turtle (Chelydra serpentina) in southeastern Missouri Timothy Charles Lescher University of Missouri-St. Louis, [email protected] Follow this and additional works at: http://irl.umsl.edu/thesis Recommended Citation Lescher, Timothy Charles, "Relative Abundance, Population Structure, and Habitat Utilization of the Alligator Snapping Turtle (Macrochelys temminckii) and Eastern Snapping Turtle (Chelydra serpentina) in southeastern Missouri" (2010). Theses. 44. http://irl.umsl.edu/thesis/44 This Thesis is brought to you for free and open access by the Graduate Works at IRL @ UMSL. It has been accepted for inclusion in Theses by an authorized administrator of IRL @ UMSL. For more information, please contact [email protected]. Relative Abundance, Population Structure, and Habitat Utilization of the Alligator Snapping Turtle (Macrochelys temminckii) and Eastern Snapping Turtle (Chelydra serpentina) in southeastern Missouri Timothy C. Lescher B.S., Biology, University of Missouri-Columbia, 2001 A Thesis Submitted to the Graduate School at the University of Missouri-Saint Louis in partial fulfillment of the requirements for the degree Master’s of Science in Biology November 2010 Advisory Committee Zuleyma Tang-Martinez, Ph.D. Chair Jeffrey T. Briggler, Ph.D. Bette A. Loiselle, Ph.D. Copyright, Timothy C. Lescher, 2010 1 TABLE -

The Common Snapping Turtle, Chelydra Serpentina
The Common Snapping Turtle, Chelydra serpentina Rylen Nakama FISH 423: Olden 12/5/14 Figure 1. The Common Snapping Turtle, one of the most widespread reptiles in North America. Photo taken in Quebec, Canada. Image from https://www.flickr.com/photos/yorthopia/7626614760/. Classification Order: Testudines Family: Chelydridae Genus: Chelydra Species: serpentina (Linnaeus, 1758) Previous research on Chelydra serpentina (Phillips et al., 1996) acknowledged four subspecies, C. s. serpentina (Northern U.S. and Figure 2. Side profile of Chelydra serpentina. Note Canada), C. s. osceola (Southeastern U.S.), C. s. the serrated posterior end of the carapace and the rossignonii (Central America), and C. s. tail’s raised central ridge. Photo from http://pelotes.jea.com/AnimalFact/Reptile/snapturt.ht acutirostris (South America). Recent IUCN m. reclassification of chelonians based on genetic analyses (Rhodin et al., 2010) elevated C. s. rossignonii and C. s. acutirostris to species level and established C. s. osceola as a synonym for C. s. serpentina, thus eliminating subspecies within C. serpentina. Antiquated distinctions between the two formerly recognized North American subspecies were based on negligible morphometric variations between the two populations. Interbreeding in the overlapping range of the two populations was well documented, further discrediting the validity of the subspecies distinction (Feuer, 1971; Aresco and Gunzburger, 2007). Therefore, any emphasis of subspecies differentiation in the ensuing literature should be disregarded. Figure 3. Front-view of a captured Chelydra Continued usage of invalid subspecies names is serpentina. Different skin textures and the distinctive pink mouth are visible from this angle. Photo from still prevalent in the exotic pet trade for C. -

UC Davis UC Davis Previously Published Works
UC Davis UC Davis Previously Published Works Title Survival, Demography, and Growth of Gopher Tortoises (Gopherus polyphemus) from Three Study Sites with Different Management Histories Permalink https://escholarship.org/uc/item/9sc747h0 Journal JOURNAL OF WILDLIFE MANAGEMENT, 78(7) ISSN 0022-541X Authors Tuberville, Tracey D Todd, Brian D Hermann, Sharon M et al. Publication Date 2014-09-01 DOI 10.1002/jwmg.773 Peer reviewed eScholarship.org Powered by the California Digital Library University of California The Journal of Wildlife Management 78(7):1151–1160; 2014; DOI: 10.1002/jwmg.773 Research Article Survival, Demography, and Growth of Gopher Tortoises (Gopherus polyphemus) from Three Study Sites with Different Management Histories TRACEY D. TUBERVILLE,1 University of Georgia’s Savannah River Ecology Lab, Drawer E, Aiken, SC 29802, USA BRIAN D. TODD, Department of Wildlife, Fish, and Conservation Biology, University of California, Davis, One Shields Ave, Davis, CA 95616, USA SHARON M. HERMANN, Department of Biological Sciences, Auburn University, Auburn, AL 36849, USA WILLIAM K. MICHENER, University Libraries, 1312 Basehart SE, Albuquerque, NM 87106, USA CRAIG GUYER, Department of Biological Sciences, Auburn University, Auburn, AL 36849, USA ABSTRACT Recovery or sustainable management of wildlife populations often entails management of habitat on which they depend. In this regard, turtles pose unique conservation challenges because of their life histories. The combination of late maturity, low survival when young, and dependence on high adult survival suggests they may be slow to respond demographically to conventional habitat management. Thus, long- term studies are necessary to understand population dynamics and recovery potential in these species. -

Snapping Turtle, Chelydra Serpentina, Overland Movements Near the Southeastern Extent of Its Range David A
Georgia Journal of Science Volume 68 No. 2 Scholarly Contributions from the Article 11 Membership and Others 2010 Snapping Turtle, Chelydra serpentina, Overland Movements Near the Southeastern Extent of its Range David A. Steen [email protected] Sean C. Sterrett Aubrey M. Heupel Lora L. Smith Follow this and additional works at: https://digitalcommons.gaacademy.org/gjs Part of the Life Sciences Commons Recommended Citation Steen, David A.; Sterrett, Sean C.; Heupel, Aubrey M.; and Smith, Lora L. (2010) "Snapping Turtle, Chelydra serpentina, Overland Movements Near the Southeastern Extent of its Range," Georgia Journal of Science, Vol. 68, No. 2, Article 11. Available at: https://digitalcommons.gaacademy.org/gjs/vol68/iss2/11 This Research Articles is brought to you for free and open access by Digital Commons @ the Georgia Academy of Science. It has been accepted for inclusion in Georgia Journal of Science by an authorized editor of Digital Commons @ the Georgia Academy of Science. 196 Steen et al.: Snapping Turtle Overland Movements SNAPPING Turtle, CHELYDRA SERPENTINA, OVERLAND MOVEMENTS NEAR THE SOUTHEASTERN EXTENT OF ITS RANGE David A. Steen1,2*, Sean C. Sterrett2, Aubrey M. Heupel2 and Lora L. Smith2 1Auburn University, 331 Funchess Hall, Auburn, Alabama, 36849 2Joseph W. Jones Ecological Research Center Route 2, Box 2324, Newton, GA 39870 Institution at which work was completed: Joseph W. Jones Ecological Research Center * Corresponding author: [email protected] ABSTRACT Terrestrial movements of turtles are of interest due to the conserva- tion implications for this imperiled group and the general lack of information on this topic, particularly in wide-ranging species. -

Bird Nests What Is a Bird's Nest?
Duke Gardens Winter Fun at Home BIRD NESTS Winter can be a good time to spot bird nests in bare tree branches that lost their leaves in the fall. Have you ever spotted any bird nests or squirrel dreys in a tree? (Drey is the word for a squirrel nest, and they usually look like a messy ball of sticks and leaves). WHAT IS A BIRD’S NEST? All birds start their life cycle as an egg. Eggs need to be protected from weather and predators. An egg also needs to stay warm from the time it is laid until it hatches. This is called the incubation period. A nest is the shelter most adult birds build to protect their eggs until A squirrel drey they hatch and to keep baby birds safe until they are ready to leave (not a bird nest!) the nest. Nests range in size, shape, location and building materials, and all of these can be clues about the kind of bird that made it. For example, bald eagle nests can be 5-6 feet across, while a cardinal’s nest is about 4 inches across. Hummingbird nests can be as small as a thimble when they are built, but they are made with materials that allow the nest to stretch as the baby birds inside it grow. Nests can be built in many places. Some birds make cavity nests inside something by finding or making an opening inside a tree, a stump or a nest box. Carolina wrens, chickadees, nuthatches, woodpeckers, and some owls are a few examples of cavity nesting birds. -

North Louisiana Refuges Complex: Freshwater Turtle Inventory
NORTH LOUISIANA REFUGES COMPLEX: FRESHWATER TURTLE INVENTORY USFWS Award No: F17PX01556 John L. Carr, Aaron C. Johnson & J. Benjamin Grizzle November 2020 ACKNOWLEDGMENTS We thank the U.S. Fish and Wildlife Service Region 4 Inventory and Monitoring Branch for USFWS Award No. F17PX01556, “Freshwater Turtle Inventory of the North Louisiana Refuges Complex”. In addition, this report incorporates data from other, complementary projects that were funded by a variety of sources. This has been done in order to provide a more fulsome picture of knowledge on the turtle fauna of the refuges within the Complex. These other projects were funded in part by the Louisiana Department of Wildlife and Fisheries and the U.S. Fish and Wildlife Service, Division of Federal Aid, through the State Wildlife Grants Program (series of projects targeting the Alligator Snapping Turtle and map turtles). Other sources include data collected for grant activities funded by USGS-BRD (Cooperative Agreement No. 99CRAG0017), funding from the Louisiana Wildlife and Fisheries Foundation beginning in 1998, funding directly from LDWF (2000-2002), The Nature Conservancy (contract #LAFO_022309), Friends of Black Bayou, the Turtle Research Fund of the University of Louisiana at Monroe Foundation, and the Kitty DeGree Professorship in Biology (2011-2017). U.S. Fish and Wildlife personnel helped facilitate our work by granting access to all parts of the refuges at various times, and providing a series of Special Use Permits over 20+ years. We acknowledge Lee Fulton, Joe McGowan, Brett Hortman, Kelby Ouchley, and George Chandler for facilitating our work on refuges over the years; in particular, we thank Gypsy Hanks for long-sustained support and cooperation, especially during the course of the current project. -

Eastern Gray Squirrel Sciurus Carolinensis
eastern gray squirrel Sciurus carolinensis Kingdom: Animalia FEATURES Phylum: Chordata The eastern gray squirrel’s head-body length is Class: Mammalia between eight and 11 inches, with its tail about the Order: Rodentia same length as the body. Its body fur is gray, and there is a border of white fur on the bushy, gray tail. Family: Sciuridae The belly fur is white, a cream line surrounds each ILLINOIS STATUS eye, and white tips are present on the back of the ears. common, native BEHAVIORS The eastern gray squirrel may be found statewide in Illinois. It lives in woods or forests that have a closed canopy, nut-bearing trees and plenty of cavity trees. As these mature forests have been destroyed in Illinois, the population of gray squirrels has declined. However, gray squirrels are common in cities. Here, they live in trees without the conditions described above. The gray squirrel eats buds, leaves, fruits, berries, fungi, pecans, acorns, hickory nuts, tree bark, walnuts and the seeds of various other trees. It stores nuts in holes in the ground. This squirrel grasps food in its front paws. It is primarily arboreal, and its large, bushy tail helps it balance while climbing and resting in trees. Urban squirrels are good at climbing brick walls and walking along wires and cables. The eastern gray squirrel does not hibernate and is active during the day year-round. It ILLINOIS RANGE may sleep for several consecutive days in winter, however. Its call is “kuk-kuk-cut-cut-cut.” This animal builds a leaf nest in the high branches of a tree but may use a tree cavity for escape from predators and poor weather and for raising its young.