Lilja Karlsdóttir-Thesis-Skemman
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geothermal Gradient Calculation Method: a Case Study of Hoffell Low- Temperature Field, Se-Iceland
International Research Journal of Geology and Mining (IRJGM) (2276-6618) Vol. 4(6) pp. 163-175, September, 2014 DOI: http:/dx.doi.org/10.14303/irjgm.2014.026 Available online http://www.interesjournals.org/irjgm Copyright©2014 International Research Journals Full Length Research Paper Geothermal Gradient Calculation Method: A Case Study of Hoffell Low- Temperature Field, Se-Iceland Mohammed Masum Geological Survey of Bangladesh, 153, Pioneer Road, Segunbagicha, Dhaka-1000, BANGLADESH E-mail: [email protected] ABSTRACT The study area is a part of the Geitafell central volcano in southeast Iceland. This area has been studied extensively for the exploration of geothermal resources, in particular low-temperature, as well as for research purposes. During the geothermal exploration, geological maps should emphasize on young corresponding rocks that could be act as heat sources at depth. The distribution and nature of fractures, faults as well as the distribution and nature of hydrothermal alteration also have to known. This report describes the results of a gradient calculation method which applied to low-temperature geothermal field in SE Iceland. The aim of the study was to familiarize the author with geothermal gradient mapping, low-temperature geothermal manifestations, as well as studying the site selection for production/exploration well drilling. Another goal of this study was to make geothermal maps of a volcanic field and to analyse if some relationship could be established between the tectonic settings and the geothermal alteration of the study area. The geothermal model of the drilled area is consistent with the existence of a structurally controlled low-temperature geothermal reservoir at various depths ranging from 50 to 600 m. -

Wonderful Fjarðabyggð You’Re in a Good Place FJARÐABYGGÐ
Wonderful Fjarðabyggð You’re in a Good Place FJARÐABYGGÐ Mj F 1 4 Information centres in Fjarðabyggð: 1 Museum House, Neskaupstaður 2 East Iceland Maritime Museum, Eskifjörður 3 Icelandic Wartime Museum, Reyðarfjörður 4 Kolfreyja Gallery, Fáskrúðsfjörður 5 Brekkan, Stöðvarfjörður 6 Sólbrekka, Mjóifjörður 2 5 The information centres in Fjarðabyggð are open in the afternoon seven days a week, June 1st to August 31st. Photographers: Kristinn Þorsteinsson, Pétur Sörensson, and others. Editor: Helga Guðrún Jónasdóttir Photo editor: Pétur Sörensson Published by: Fjarðabyggð municipality, 2014 Design and layout: Héraðsprent, www.heradsprent.is 3 6 No responsibility is taken for the reliability of information on shopping and other services. Hoffell, Fáskrúðsfjörður Mj F A hearty welcome to Fjarðabyggð! Our community’s magnificent mountains and picturesque fjords are just part of what Fjarðabyggð has to offer. Equally memorable to those who visit are the society Sandfell, Fáskrúðsfjörður and culture of our seaside villages, each nestling with its own spirit and character along Iceland’s easternmost coast. Every year, the Fjarðabyggð combination of landscape, history and personalities attracts more visitors. You can easily find the hotel or guest house best suited to your desires, or choose one of Fjarðabyggð’s six campgrounds. You’ll also find plenty of choices for recrea- tion, in a municipality where both mountain slopes and seashores lie just beyond your doorstep. No matter where else you’re heading in East Iceland, Fjarðabyggð will be worth -

Höfn Is Known Nearby Are Dysjarklettur And, Below the Main Road, Fimmhundraðadý
farm Dynjandi, a little west of the road up to the Almannaskarð pass. 15. Hoffellsdalur been appointed to consolidate royal power in Iceland. In 1433 Teitur investigated in 1902 by Daniel Bruun, who wrote: 'It was shown beyond fishing vessels land rich catches throughout the year; Höfn is known Nearby are Dysjarklettur and, below the main road, Fimmhundraðadý. Starting point: Below the farm at Hoffell and others attacked the bishop at the cathedral at Skálholt, stuck him in doubt that a man had been buried towards the south of the ridge, 3-4 especially for lobsters in spring and herring in autumn. The harbour About half way along is the waterfall Bergárfoss (see route 5). Route description: Fairly easy day's walk, mostly quite even; about a sack, and drowned him in a nearby river. feet above the floodplain. Other finds included a breastplate and traces bustles with life and is a great attraction for tourists. Walking trails in Nes 12 km to the sheltered dell at Dalsstafn at the head of the valley, where of a thin layer of coal under and around the bones. In an extension to the north there was a horse burial, or perhaps just a horse's head. There The rich farmlands around Höfn produce potatoes, milk and lamb, as 7. Laxárdalur there is glacial debris from when the ice extended this far down until about a century ago. Start off over the mudflats below Hoffell farm were also several horse's teeth. There are no records of brooches well as some beef and pork. -

THE Orlefajokull ERUPTION of 1362
ACTA NATURALIA ISLANDICA VOL. II. - NO.2. THE ORlEFAJOKULL ERUPTION OF 1362 BY SIGURDUR THORARINSSON WITH 1 PLATE AND 31 FIGURES IN THE TEXT NATTURUGRIPASAFN ISLANDS MUSEUM RERUM NATURALIUM ISLANDIAE REYKJAVIK 1958 fsatoldarprenlsml6ja h.t. CONTENTS PAGE INTRODUCTORY 5 ORiEFAJOKULL. SHAPE AND SIZE.................................... 5 THE GEOLOGY OF ORiEFAJOKULL 8 THE GEOLOGICAL STRUCTURE AND STRATIGRAPHY OF THE ORiEFA- JOKULL MASSIF 10 THE SETTLEMENT AT TEE FOOT OF ORiEFAJOKULL 17 THE GREAT ERUPTION 25 THE JOKULHLAUPS OF 1362 29 THE EVIDENCE OF THE TEPHRA LAyERS........................... 37 Layers from Katla 43 The Eldgja Layers 47 Hekla 1845 47 Layers from Grimsvotn 48 The light Hekla Layers 48 Askja 1875 49 Layer G 50 Layer "a" 50 o 1362 57 Notes on Table 3 59 The Depth of 0 1362 7Q The Chemical Composition and other Properties of the 1362.tephra 71 THE TYPE OF ERUPTION AND THE ERUPTION CyCLE............... 72 THE THICKNESS AND EXTENSION OF 0 1362. THE ISOPACHYTE MAP 75 THE THICKNESS AND VOLUME OF THE TEPHRA LAYER WHEN FRESHLY FALLEN .................................... .. 79 THE PROBABLE WEATHER SITUATION DURING THE TEPHRA FALL 81 THE EFFEC'l' OF THE 1362-ERUPTION ON THE SHAPE OF ORiEFA- JOKULL 84 COMPARISON WITH THE ANNALS 84 ON THE FATE OF THE INHABITANTS OF HEmAD ;.... 86 FOR HOW LONG DID THE SETTLEMENT REMAIN ABANDONED? 88 1 SUMMARY 93 i~ REFERENCES 96 INTRODUCTORY The LandnamabOk tells us that when Ing61fur Arnarson, "the first settler" in Iceland, went to that country for the second time, in 874 A.D., with a view to making his abode there, he threw overboard his high seat pillars for luck, and vowed that wherever he might find them washed ashore, he would make his home. -

Biomimicry in Iceland: Present Status and Future Significance
Biomimicry in Iceland: Present Status and Future Significance Sigríður Anna Ásgeirsdóttir Faculty of Industrial Engineering, Mechanical Engineering and Computer Science University of Iceland 2013 i Biomimicry in Iceland: Present Status and Future Significance Sigríður Anna Ásgeirsdóttir 30 ECTS thesis submitted in partial fulfillment of a Magister Scientiarum degree in Environment and Natural Resources Advisors Sigurður Brynjólfsson Brynhildur Davíðsdóttir External examiner Guðmundur Valur Oddsson Faculty of Industrial Engineering, Mechanical Engineering and Computer Science University of Iceland Reykjavik, November 2013 i Biomimicry in Iceland: Present Status and Future Significance Short title: Biomimicry in Iceland 30 ECTS thesis submitted in partial fulfillment of a Magister Scientiarum degree in Environment and Natural Resources Copyright © 2013 Sigríður Anna Ásgeirsdóttir All rights reserved Faculty of Industrial Engineering, Mechanical Engineering and Computer Science University of Iceland Hjarðarhagi 6 107, Reykjavik Iceland Telephone: 525 4000 Bibliographic information: Sigríður Anna Ásgeirsdóttir, 2013, Biomimicry in Iceland: Present Status and Future Significance, Master’s thesis, Faculty of Industrial Engineering, Mechanical Engineering and Computer Science, University of Iceland, pp. 75. Printing: Háskólaprent Reykjavik, Iceland, November 2013 ii Abstract The word biomimicry is derived from bios = life and mimesis = imitation. Biomimicry is a relative new field that studies nature´s designs and mimics them to create sustainable approaches for technical designs and innovation. The founder of biomimicry, the American biologist Janine Benyus, emphasis that answers to many of the environmental challenges we are facing today have already been provided by sustainable solutions that living organisms have developed for over 3.8 billion years. This thesis introduces the principles of biomimicry and its relation to other green and bio-inspired disciplines, followed by analyses of the status of biomimicry in Iceland. -

PETROLOGICAL STUDIES of the GEITAFELL CENTRAL VOLCANO, S.E. ICELAND JOFIANNA M THORLACIUS Thesis Submitted for the Degree Of
PETROLOGICAL STUDIES OF THE GEITAFELL CENTRAL VOLCANO, S.E. ICELAND JOFIANNA M THORLACIUS Thesis submitted for the degree of Master of Philosophy University of Edinburgh 1991 DECLARATION I declare that this thesis is my own work except where otherwise stated. Jóhanna 11 Thorlacius [Ii ABSTRACT The Geitafell central volcano was active 6-5 my ago and involved a tholeiitic suite comparable to those of other Tertiary volcanic centres in Iceland. Geochemical evidence suggests that the volcano was located in an axial rift-zone, supplied by magmas derived from large degrees of melting in a relatively garnet-rich mantle source. The magmas are inferred to have collected and fractionated in a magma-chamber at slightly deeper crustal levels than those of the present axial rift-zone. Magmatic activity was for a long period characterised by the extrusion of relatively evolved, small volume, phenocryst-poor basaltic lavas, grading into basaltic andesites; however, early events included the mixing of basic and acid magma resulting in the production of composite units. A shorter period of intense intrusive activity followed, which saw the emplacement of less evolved, more porphyritic basalts as sheets and dykes with intermittent intrusive phases producing diorites and quartz-diorites. The extrusive rocks that may have been fed by the sheets and dykes have been removed by erosion, except for late-stage rhyolite lavas fed by relatively thick micro-granitic dykes. A phase involving the intrusion and extrusion of highly porphyritic magma was pene- contemporaneous with the rhyolitic phase; collectively these represent the last magmatic activity related to the Geitafell complex. Gabbroic intrusions of irregular (non-tabular) shape are related to the suite of lavas, sheets and dykes; they represent, to varying degrees, cumulates of plagioclase, pyroxene and FeTi-oxides ± divine. -

Changes in the Southeast Vatnajökull Ice Cap, Iceland, Between ∼1890
The Cryosphere, 9, 565–585, 2015 www.the-cryosphere.net/9/565/2015/ doi:10.5194/tc-9-565-2015 © Author(s) 2015. CC Attribution 3.0 License. Changes in the southeast Vatnajökull ice cap, Iceland, between ∼ 1890 and 2010 H. Hannesdóttir, H. Björnsson, F. Pálsson, G. Aðalgeirsdóttir, and Sv. Guðmundsson Institute of Earth Sciences, University of Iceland, 101 Reykjavík, Iceland Correspondence to: H. Hannesdóttir ([email protected]) Received: 8 August 2014 – Published in The Cryosphere Discuss.: 5 September 2014 Revised: 7 February 2015 – Accepted: 11 February 2015 – Published: 19 March 2015 Abstract. Area and volume changes and the average majority of glaciers worldwide have been losing mass during geodetic mass balance of the non-surging outlet glaciers of the past century (Vaughan et al., 2013), and a number of the southeast Vatnajökull ice cap, Iceland, during different studies have estimated the volume loss and the mass balance time periods between ∼ 1890 and 2010, are derived from for the post-LIA period by various methods (e.g. Rabatel a multi-temporal glacier inventory. A series of digital et al., 2006; Bauder et al., 2007; Knoll et al., 2008; Lüthi elevation models (DEMs) (∼ 1890, 1904, 1936, 1945, 1989, et al., 2010; Glasser et al., 2011). Knowledge of the ice 2002, 2010) are compiled from glacial geomorphological volume stored in glaciers at different times is important features, historical photographs, maps, aerial images, DGPS for past, current and future estimates of sea-level rise and measurements and a lidar survey. Given the mapped basal water resources. More than half of the land ice contribution topography, we estimate volume changes since the end of the to sea-level rise in the 20th century comes from ice caps Little Ice Age (LIA) ∼ 1890. -

2015-07-1-3 Travel Report 2015-07-1-3 Stokksnes
1.7. 2015 Stokksnes, Höfn, Hoffell We braved the cold and rainy weather and did a walk in Stokksnes not far from Höfn. Black lava sand, low clouds and with the fog it was a special scenery. If only the wind would not be so strong and so cold… On the way we learned how to set the grass. After braving the cold for so long and being really cold, we as usual had to reward ourselves ;-) 1 Fully recharged we experienced our first glacier, the Hoffellsjökull. To get the best view, we had to climb some quite challenging steeps! There was no wind, we were all by ourselves, really impressive. 2 On the way out, we passed by the natural hot springs of Hoffell II. We couldn’t give it a miss, although it was again quite tough to take off all your clothes and go out in a swimsuit at 9C outside temperature. But these pools were really hot, so the starting rain was actually refreshing. What followed was the usual search for a camping spot. Not far from Hoffell we discovered a small street, which was covered in fog. We followed the dam into the nowhere. On the right the river, on the left wide space, we could’t see much else… Had an eery feeling… Except for two reindeer. At the end of the road we just stopped for the night. 2.7.2015-07-02 Flaajökull, Skalafell The next morning it all looked very different! The fog was gone and to our surprise we had prime glacier view. -
Highlands and Lowlands
Hosting you since 1937 Self Drive 9 days Highlands and Lowlands Námafjall Welcome to Iceland! To make your stay in Iceland more enjoyable, we would like to draw your attention to the following: △ This booklet contains a standard itinerary. If you have Sat Nav added nights, please note that the itinerary has not been customized to show such amendments. △ This itinerary is a guideline for your travel in Iceland. The Speed Limit route may vary according to your overnight stay and road conditions, especially during April/May and September/ October. Seat Belts △ Even with the long daylight, you will not be able to visit every nook and cranny nor enjoy all the fun things sug- gested. Please choose between the sites mentioned Road Map depending on your interest as time limits your possibilities. △ The kilometer/mile distance per day is not precise. The distance may vary depending on the location of your Bin the Litter accommodation and the detours you take each day. △ On your accommodation itinerary you will find a detailed address list, describing how to reach your accommoda- One Lane Bridge tions. The accommodation itinerary also has a reference number, which is valid as your voucher. Official hotel check-in time is 14:00-18:00. If you arrive any later, it’s Watch out for Animals courteous to notify the hotel. △ Breakfast is always included during your stay, but no other meals. Many of the rural hotels and guesthouses offer a Gravel Roads nice dinner at a reasonable price. 2 Self Drive Highlands and Lowlands A few favourites from the trip -
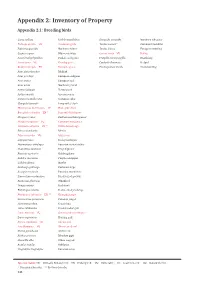
Appendix 2: Inventory of Property
Appendix 2: Inventory of Property Appendix 2.1: Breeding birds Gavia stellata Red-throated diver Oenanthe oenanthe Northern wheatear Podiceps auritus VU Slavonian grebe Turdus merula * Common blackbird Fulmarus glacialis Northern fulmar Turdus iliacus European redwing Cygnus cygnus Whooper swan Corvus corax VU Raven Anser brachyrhynchus Pink-footed goose Fringilla montifringilla Brambling Anser anser VU Graylag goose Carduelis flammea Redpoll Branta leucopsis EN Barnacle goose Plectrophenax nivalis Snow bunting Anas platyrhynchos Mallard Anas penelope European widgeon Anas crecca European teal Anas acuta Northern pintail Aythya fuligula Tufted duck Aythya marila Greater scaup Somateria mollissima Common eider Clangula hyemalis Long-tailed duck Histrionicus histrionicus LC Harlequin duck Bucephala islandica EN * Barrow’s Goldeneye Mergus serrator Red-breasted merganser Mergus merganser VU Common merganser Haliaeetus albicilla EN ** White-tailed eagle Falco columbarius Merlin Falco rusticolus VU Gyrfalcon Lagopus muta Rock ptarmigan Haematopus ostralegus Eurasian oystercatcher Charadrius hiaticula Ringed plover Pluvialis apricaria Golden plover Calidris maritima Purple sandpiper Calidris alpina Dunlin Gallinago gallinago Common snipe Scolopax rusticola Eurasian woodcock Limosa limosa islandica Black-tailed godwit Numenius phaeopus Whimbrel Tringa totanus Redshank Phalaropus lobatus Red-necked phalarope Phalaropus fulicarius EN ** Gray phalarope Stercorarius parasiticus Parasitic jaeger Stercorarius skua Great skua Larus ridibundus Black-headed -

The Formation of a Palagonite Breccia Mass Beneath a Valley Glacier in Iceland of OROE PATRICI LF ONARD WALKER & DAVID HENRY BLAKE
The formation of a palagonite breccia mass beneath a valley glacier in Iceland OF OROE PATRICI LF ONARD WALKER & DAVID HENRY BLAKE CONTENTS I Introduction 45 ~, Geological setting 47 3 Field relationships and structure 48 4 Petrology 5 I 5 Discussion of the evidence 52 (a) The glacier in occupation 5~ (b) Mode of emplacement 53 (c) Location of the eruptive source 54 (d) Other palagonite breccias . 54 6 Appendix: other valley-fillings in south-east Iceland 57 7 References 57 Plat es 3-6 between 58 and 59 SUMMARY A mass of palagonite breccia, with associated original volume must have been several times palagonite tufts, basalt pillows, and large bodies this) and the basalt flowed certainly for 22 km, of columnar basalt, is described from south- and probably for 35kin, beneath the ice. It is eastern Iceland. It rests upon a glacially believed to have flowed through a central striated surface, with or without a thin inter- conduit along the valley bottom, and the vening tillite layer. The mass is probably of breccia and contained pillows mainly developed early Pleistocene age and the evidence shows by escape of the basalt upwards and sideways that the basalt which gave rise to it flowed from this conduit into the glacier or its melt- down a valley beneath a valley-glacier. The waters. mass has a present volume of I'5km 3 (the 1. Introduction P A L A O O N I T E breccias and tufts make up some of the most conspicuous mountains in Iceland, and account for more than one-eighth of the area of the country. -

What to Do in and Around Höfn HÖFN Í HORNAFIRÐI
What to do in and around Höfn HÖFN Í HORNAFIRÐI Höfn í Hornafirði is a thriving community close to the largest glacier in Europe, Vatnajökull, in the south eastern part of Iceland. The district’s most densely populated area is Höfn. Höfn’s growth in the 20th century In this region you are located at the from a small village to today’s town base of Vatnajökull, and the scenery was a result of the development of is breathtaking. Vatnajökull National a relatively diverse fishing industry. Park, the largest national park in The landed catch is now more varied Europe, is situated in the community. than in other fishing towns, including In the last few decades tourism has high-value species such as the Norway grown enormously and has in fact lobster (Nephrops norvegicus). In become a major economic sector in recent years the town has promoted the community. Diverse services are lobster as a central part of its image, provided including hotels, camping for instance with a “lobster festival” sites, restaurants, shops, swimming that is held each summer. Höfn actually pools, golf courses, marked hiking means harbour, though repeated trails and various museums, such as dredging has proved necessary to keep maritime museum, two national park the entrance of the harbour safe. visitor centres and the centre of the great writer Þórbergur Þórðarson. HÓTEL HÖFN RING ROAD NR. 1 1 ÆGISSÍÐUFOSS 17 JÖKULSÁRLÓN WATERFALL DIAMOND BEACH 2 MOUNTAIN DÍMON 18 THE ÞÓRBERGUR CENTRE 3 SELJALANDSFOSS 19 HOFFELLSJÖKULL & GLJÚFRABÚI 20 LÓNSÖRÆFI 4 KATLA AKUREYRI 21 HÖFN