Inherently Slow Growth in Two Caribbean Epiphytic Species: a Demographic Approach
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genome Relationships in the Oncidium Alliance A
GENOME RELATIONSHIPS IN THE ONCIDIUM ALLIANCE A DISSERTATION SUBMITTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF HAWAII IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN HORTICULTURE MAY 1974 By Uthai Charanasri Dissertation Committee: Haruyuki Kamemoto, Chairman Richard W. Hartmann Peter P, Rotar Yoneo Sagawa William L. Theobald We certify that we have read this dissertation and that in our opinion it is satisfactory in scope and quality as a dissertation for the degree of Doctor of Philosophy in Horticulture. DISSERTATION COMMITTEE s f 1 { / r - e - Q TABLE OF CONTENTS Page LIST OF T A B L E S .............................................. iii LIST OF ILLUSTRATIONS...................................... iv INTRODUCTION ................................................ 1 REVIEW OF LITERATURE.................. 2 MATERIALS AND M E T H O D S ...................................... 7 RESULTS AND DISCUSSION ....................................... 51 Intraspecific Self- and Cross-Pollination Studies ........ Intrasectional Cross Compatibility within the Oncidium G e n u s ............................... 58 Intersectional and Intergeneric Hybridizations .......... 80 Chromosome Numbers ..................................... 115 K a r y o t y p e s ............................................ 137 Meiosis, Sporad Formation, and Fertility of Species Hybrids ............................. 146 Morphology of Species and Hybrids ..................... 163 General Discussion ................................... 170 SUMMARY -

Oncidium Intergenerics
NEWSLETTER January 2020 Volume 15 Issue #1 CLUB NEWS January 7, 2020 Monthly SAOS Meeting by Janis Croft Welcome and Thanks. President Tom Sullivan opened the meeting at 7:00 pm with a 72 attendees. Events VP, Dianne Batchhelder thanked Dottie Your catasetums are likely sleeping now so just look in for bringing in her Chocolate on them every week looking for signs of the new growth Pudding Cake and then which is the time to repot, if they need repotting this year. thanked all who volunteered If you need any potting supplies, email info@ and worked so hard to make staugorchidsociety.org and we will have it ready for you Philip Hamilton our December holiday party at the next meeting. Potting Mix and Fertilizers, $5 each; a success including Mary Durable Plant Tags, $5 for 30 tags; 2020 Calendars, $15 Ann Bell for her Pork Roast (Dianne can provide the recipe) or 2 for $25; Slotted Orchid Pots, 3 to 6 inch pots, $1 to $4 and Susan Smith for her lasagna and Yvonne and Bob for each. washing all the tablecloths! In addition, thanks also went Linda Stewart asked all of the January birthday people to Joey, Celia and Dottie for setting up the refreshments to raise their hands to received their free raffle ticket. and Tom and Bob for set up and Charlie and Doug for Then she announced that if you know of anyone in need breakdown. of a cheering up or a get well card, email her at info@ Membership VP Linda Stewart announced our six new staugorchidsociety.org. -

University of Florida Thesis Or Dissertation Formatting
A MONOGRAPH OF THE GENUS LOCKHARTIA (ORCHIDACEAE: ONCIDIINAE) By MARIO ALBERTO BLANCO-COTO A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2011 1 © 2011 Mario Alberto Blanco-Coto 2 To my parents, who have always supported and encouraged me in every way. 3 ACKNOWLEDGMENTS Many individuals and institutions made the completion of this dissertation possible. First, I thank my committee chair, Norris H. Williams, for his continuing support, encouragement and guidance during all stages of this project, and for providing me with the opportunity to visit and do research in Ecuador. W. Mark Whitten, one of my committee members, also provided much advice and support, both in the lab and in the field. Both of them are wonderful sources of wisdom on all matters of orchid research. I also want to thank the other members of my committee, Walter S. Judd, Douglas E. Soltis, and Thomas J. Sheehan for their many comments, suggestions, and discussions provided. Drs. Judd and Soltis also provided many ideas and training through courses I took with them. I am deeply thankful to my fellow lab members Kurt Neubig, Lorena Endara, and Iwan Molgo, for the many fascinating discussions, helpful suggestions, logistical support, and for providing a wonderful office environment. Kurt was of tremendous help in the lab and with Latin translations; he even let me appropriate and abuse his scanner. Robert L. Dressler encouraged me to attend the University of Florida, provided interesting discussions and insight throughout the project, and was key in suggesting the genus Lockhartia as a dissertation subject. -

November 1992 Newsletter
Odontoglossum Alliance shortened cross to flower cycle. He illustrated Odontoglossum his techniques with excellent visual material including many suggestions for proceeding from Alliance Meeting the flask through community pot to flowering. Bob has promised to write up his talk to be printed in a future newsletter. a Success The John Day scrapbooks were a monumental The Odontoglossum Alliance held its annual achievement produced in the period of mid to late meeting in conjunction with the Eastern Orchid ISOO’s. Mr. Michael Tibbs of Stonehurst Congress in Boston, Massachusetts on 23 Nurseries had completed a study of these books October. Bob Dugger, Program Chairman, now located at Kew Gardens. Michael extracted a introduced each of the four speakers. Allan large amount of interesting material on Moon, Curator, Eric Young Orchid Foundation odontoglossums produced by Day. Michael’s described their efforts to create albino illustrations and talk was interesting and odontoglossums. This work had started under informative. Day produced colored flowers, in Mr. Charlesworth, but had lain in neglect water color with great detail, but often left the following his death in 1920. The Foundation had rest of the plant vaguely sketched. Day also pursued a course of producing albino flowers included letters, comments, and clippings from using the original Charlesworth progress and gardeners publications. combining it with recent chromosome count The meeting was attended by about 150 people work of Don Wimber producing amazing and who where very enthusiastic about the high beautiful results. Allan showed slides of large quality of the program. Following the lecture pure white odontoglossums, white with yeUow series, the Alliance held an auction of fine markings, and clear yellows. -

Orchidaceae Flora of Joinville, Santa Catarina, Brazil Flora De Orchidaceae De Joinville, Santa Catarina, Brasil
Acta Biológica Catarinense 06 Jan-Jun;3():36-48 Orchidaceae Flora of Joinville, Santa Catarina, Brazil Flora de Orchidaceae de Joinville, Santa Catarina, Brasil Werner Siebje MANCINELLI,3 & Karin ESEMANN-QUADROS ABSTRACT Recebido: 7 jan. 06 The objective of this work was to conduct a floristic study of Orchidaceae in the municipality Aceito: 9 mar. 06 of Joinville (Santa Catarina, Brazil), which occurred from 2006 to 2015. The municipality is mainly covered with Atlantic Forest between 0 and 1,335 m elevation. For the family, 211 species within 90 genera were recorded. The richest genera are Epidendrum (17 spp.), Pabstiella (15 spp.), Acianthera (14 spp.), Anathallis (8 spp.) and Octomeria (8 spp.). The majority of the species are epiphytes (82%), 11% are terrestrial, and the remaining 7% are hemi-epiphytes, rupicolous or myco-heterotrophic. Two species, Grobya guiselii and Homalopetalum joinvillense, are known only from Joinville. Keywords: Atlantic Forest; Floristic; Orchids; South Brazilian. RESUMO Este trabalho teve como objetivo realizar o estudo florístico de Orchidaceae em Joinville (Santa Catarina – Brasil), que ocorreu entre 2006 e 2015. O município é caracterizado principalmente pela mata atlântica nativa, que pode ser encontrada entre as altitudes de 0 e 1.335 m. A família possui 211 espécies distribuídas em 90 gêneros. Os gêneros mais ricos são Epidendrum (17 spp.), Pabstiella (15 spp.), Acianthera (14 spp.), Anathallis (8 spp.) e Octomeria (8 spp.). A maioria das espécies ocorre como epífita (82%), 11% são terrícolas, e hemiepífitas, rupícolas e mico-heterotróficas representam 7%. Até o momento, duas espécies são conhecidas apenas para Joinville: Grobya guiselii e Homalopetalum joinvillense. -

Eliokarmos Humanii (Hyacinthaceae, Ornithogaloideae), a New Species from Namaqualand in South Africa and a New Combination in the Genus
Phytotaxa 474 (1): 087–092 ISSN 1179-3155 (print edition) https://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2020 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.474.1.9 Eliokarmos humanii (Hyacinthaceae, Ornithogaloideae), a new species from Namaqualand in South Africa and a new combination in the genus MARIO MARTÍNEZ-AZORÍN1,3*, MANUEL B. CRESPO1,4, MARÍA ÁNGELES ALONSO-VARGAS1,5 & MICHAEL PINTER2,6 1 Depto. de Ciencias Ambientales y Recursos Naturales (dCARN), Universidad de Alicante, P. O. Box 99, E-03080 Alicante, Spain. 2 Institute of Biology, NAWI Graz, Division Plant Sciences, Karl-Franzens University Graz, Holteigasse 6, 8010 Graz, Austria. 3 �[email protected]; https://orcid.org/0000-0002-2605-9575 4 �[email protected]; https://orcid.org/0000-0002-3294-5637 5 �[email protected]; https://orcid.org/0000-0003-3768-9203 6 �[email protected]; https://orcid.org/0000-0002-6055-6989 *Author for correspondence. Abstract A new species of the southern Africa endemic genus Eliokarmos, that includes the well-known chincherinchees, is described from the vicinity of Kotzesrus, Northern Cape Province of South Africa. Eliokarmos humanii sp. nov. is unique in the genus based on its single, slightly fleshy, suborbicular, convex leaf with ciliate margin, and the short subspiciform inflorescence with almost sessile flowers. A complete description is presented for this species, and data on morphology, ecology, and distribution are reported. In addition, Ornithogalum richtersveldensis, recently described from northwestern South Africa, is transferred to Eliokarmos based on its morphology and biogeography, and a new combination is presented for this species in the latter genus. -
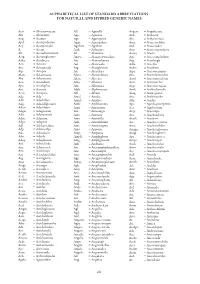
Orchid Name Abbreviations List
ALPHABETICAL LIST OF STANDARD ABBREVIATIONS FOR NATURAL AND HYBRID GENERIC NAMES Acw. = Aberconwayara All. = Aganella Angcst. = Angulocaste Abr. = Aberrantia Agn. = Aganisia Ank. = Anikaara Acp. = Acampe Agt. = Aganopeste Akr. = Ankersmitara Apd. = Acampodorum Agsp. = Agasepalum Anct. = Anoectochilus Acy. = Acampostylis Agubata = Agubata Atd. = Anoectodes A. = Aceras Aitk. = Aitkenara Ano. = Anoectogoodyera Ah. = Acerasherminium Al. = Alamania Anota = Anota Actg. = Aceratoglossum Agwa. = Alangreatwoodara Ayp. = Ansecymphyllum Acba. = Acinbreea Atc. = Alantuckerara Asg. = Anselangis Acn. = Acineta Aat. = Alaticaulia Aslla. = Ansellia Ain. = Acinopetala Atg. = Alatiglossum Asdm. = Ansidium Aip. = Aciopea Alc. = Alcockara Arpt. = Anteriocamptis Akm. = Ackermania Alxra. = Alexanderara Ahc. = Anterioherorchis Aks. = Ackersteinia Alcra. = Aliceara Atml. = Anteriomeulenia Aco. = Acoridium Alna. = Allenara Antr. = Anteriorchis Apa. = Acrolophia Aln. = Allioniara Atsp. = Anterioserapias Aro. = Acronia Alph. = Alphonsoara Anth. = Anthechostylis Acro. = Acropera Alv. = Alvisia Antg. = Antheglottis Ada = Ada Amal. = Amalia Anr. = Antheranthe Adh. = Adachilum Amals. = Amalias Alla. = Antilla Adg. = Adacidiglossum Amb. = Amblostoma Apr. = Apoda-prorepentia Adcm. = Adacidium Amn. = Amenopsis Aea. = Appletonara Adgm. = Adaglossum Am. = Amesangis Arcp. = Aracampe Adn. = Adamantinia Ams. = Amesara Ara. = Arachnadenia Adm. = Adamara Ame. = Amesiella Arach. = Arachnis Adps. = Adapasia Aml. = Amesilabium Act. = Arachnocentron Adl. = Adelopetalum Ami. = Amitostigma -

Alphabetical One-Table List of Genera and Intergeneric Combinations
ALPHABETICAL ONE-TABLE LIST OF GENERA AND INTERGENERIC COMBINATIONS (with parentage, where applicable, and standard abbreviations) This list contains the names and intergeneric combinations of all genera, including hybrid genera, which occur in current use in orchid hybrid registration as at 31st. December 2007, regardless of whether they appear in the main list of this volume. The arrangement of the entries is in One-Table format, and for intergeneric combinations is as follows: Under the name of each natural genus is entered (inset) each combination in which it occurs together with the name (following the = sign) of the intergeneric name which represents that combination. Each such combination appears once under the name of the natural genus concerned, the entries being in the alphabetical sequence of the remaining “constituent” genera which, in any entry, are themselves alphabetically arranged. Thus in the course of the whole list each bigeneric combination occurs twice, each trigeneric combination occurs three times, and so on. For example, in addition to the entry of BRASSOLAELIOCATTLEYA in bold type in its own alphabetical position, the combination for Brassolaeliocattleya appears three times, inset: Once under BRASSAVOLA as × Cattleya × Laelia = Brassolaeliocattleya Once under CATTLEYA as × Brassavola × Laelia = Brassolaeliocattleya Once under LAELIA as × Brassavola × Cattleya = Brassolaeliocattleya ACAMPE (Acp.) = Natural genus × Arachnis ................... =Aracampe × Armodorum .................. =Acampodorum × Rhynchostylis -

Orchid Hybrids Exempted from CITES Annex 1
CITES Identification Manual Orchid hybrids exempted from CITES Annex 1 CITES Identification Manual Orchid hybrids exempted from CITES "Artificially propagated specimens of orchid hybrids of the taxa listed below are not subject to the pro- visions of the Convention when they: (a) are traded in flowering state, i.e. with at least one open flower per specimen, with reflexed petals; (b) are professionally processed for commercial retail sale, e.g. labelled with printed labels and packaged with printed packages; (c) can be readily recognized as arti- ficially propagated specimens by exhibiting a high degree of cleanliness, undamaged inflorescences, intact root systems, and general absence of damage or injury that could be attributable to plants origi- nating in the wild; (d) plants do not exhibit characteristics of wild origin, such as damage by insects or other animals, fungi or algae adhering to leaves, or mechanical damage to inflorescences, roots, leaves, or other parts resulting from collection; and (e) labels or packages indicate the trade name of the specimen, the country of artificial propagation or, in case of international trade during the produc- tion process, the country, where the specimen was labelled and packaged; and labels or packages show a photo of the flower, or demonstrate by other means the apropriate use of labels and packages in an easily verifiable way. Plants not clearly qualifying for the exemption must be accompanied by appropriate CITES documents." Cymbidium Interspecific hybrids within the genus and intergeneric -

Brassavola Nodosa Program Chair All En Cogar Had Not Gotten a Finn Wlvw.Orchid'i.Com Commitment from His I'ntended August Speaker at the Kid
Amelian Ordtki ~ DAS5A OI.t!nruWMd ~ ~ ScrwIoooAwwd An Affiliate of the American Orchid Society FORT LAUDERDALE ORCHID SOCIETY August, 2010 th Program for August 9 Classifi cation and more from Wikimedia photo credit. Eric Fang Brassavola nodosa Program chair All en Cogar had not gotten a finn wlVw.orchid'i.com commitment from his i'ntended August speaker at the kid. Plantae time the newsletter needed to get to the printer. No p. Angiosper m problem, our wonderful website wi ll have the news, c. Monocot Yo u can bet, based on our great Ju ly program, that the o. Asparagaies wi ll be one to attend. f. Orchidacea sub f. Epidendoideae On Classification tribe Epidendreae sub trihe Laeliinac Use this pneumonic to remember basic classificati on: Alliance- Cattleya "Kathy poured/dumped coffee on father's grey suit" g. lJrassavo/a Kathy = kingdom s. not/osa poured/dumped = phylum fo r animals, r.; division tor plants B. nodosa was brought to Holl and in 1698 from the coffee = class Caribbean is land, Curacae. Wikimedia listed these on = ord er ve ry outstanding Brassavola hybrids: fa ther's = family grey suit = genus and species. B. Little Stars (B. nodosa x B. sublilofolia) Man 's classification is: Animal kingdom, Chordate phvlum (embryonic gill slits and notochord), mammal Rhynchovola David Sanders (B. cucullata x class (hai r and mi lk), primate order (finger nails, not RhYllchovola digbyana) hoofs or claws), hominidae family (separates liS from apes), homo genus (cave and modem man) and sapiens Be. Yell ow Bird (Bc. Richard Muell er x B. -

Alaris Capture Pro Software
J Odontoglossum Alliance Newsletter these events will be detailed in the November 1994 Programs & 1993 newsletter. I urge you to mark your Meetings calendars, and schedule one or both of these interesting and important Odontoglossum Alliance supported events. Send in your Santa Santa Barbara Barbara registration now. Meeting & Program , The Odontoglossum Alliance will hold its annual meeting in Sante Barbara, California on 11 First British March 1994. This meeting will be held in Odontoglossum conjunction with the Cymbidium Society, holding its meeting on 12 March 1993, and the Alliance Show Santa Barbara Orchid Show 11-13 March 1993. Steve Beckendorf will lead off the speakers The British Odontoglossum Alliance will hold its program with "Odontoglossum Alliance Plant first show and lectures session on 25 September Genetics". This meeting is described in detail 1993 sta^ng at 11:00 am. The locaflorTisr later in the newsletter. Bridgewater Hotel 2110 Warwick Road Greater New York Knowle, Solihull, West Midlands There are two lectures planned and a small show Orchid Show concluding at 4:30 pm. For further details Program contact the Chairman, British Odontoglossum The Odontoglossum Alliance will sponsor a Alliance, Mr. Allan Long, Director, Mansell & program of three speakers to be held at the Hatcher, Ltd., Cragg Wood Nurseries, Greater New York Orchid Show on 23-27 March Woodlands Drive, Rawdon, Leeds, LS19 6LQ, 1994. The Eastern Orchid Congress will be held Telephone 0532 502016. in conjunction with the Greater New York Orchid Show. The speakers program will have Maurice Election of Directors LeCoufle, of Vacherot & LeCoufle speaking on "Odontoglossum Growing in Northern France The following have been elected to be the Board Since 1886", Michael Tibbs of the Exotic Plant of Directors of the Odontoglossum Alliance. -

Aspasia Lunata (Lindley)
Aspasia lunata (Lindley) Bert Van Zuylen – Patrick Mannens Familie: Cymbidieae onderfamilie: Oncidiinae Aspasia is een klein geslacht van waarschijnlijk niet meer dan 10 soorten. Het zijn middelgrote planten met aantrekkelijke bloemen die lang mooi blijven. Qua bouw lijken ze op Oncidium - soorten, een geslacht waaraan ze ook nauw verwant zijn. In 1833 werd het geslacht voor het eerst beschreven door John Lindley, maar waarom hij deze naam koos is niet helemaal duidelijk. Hij gebruikte ofwel het Griekse woord "aspasios" (mooi, glad), ofwel vernoemde hij het geslacht naar Aspasia, de knappe vrouw van Pericles. Maar omdat beide woorden schoonheid omschrijven zijn beide verklaringen goed. O.V.V. - Orchiteek Pagina 1 Deze sympodiale epifieten (die soms ook lithofitisch groeien) hebben afgeplatte, vaak gegroefde pseudobulben die soms op een korte steel staan. Elke bulbe draagt 4 of meer bladeren die de bulbe aan de onderkant omsluiten en dus zo die korte steel kunnen verbergen en verder draagt elke bulbe één of twee bladeren op de top. De zachte, leerachtige, speervormige bladeren kunnen tot 30 cm lang worden en tot 5 cm breed. Een of meerdere bloemstengels, die wel 25 cm lang kunnen worden, verschijnen vanuit de bladoksels onder aan de bulbe. De dominante bloemkleuren zijn wit, lavendel en bruin. De lengte van de bloemen is groter dan de breedte en kan variëren van 4-7,5 cm. De drie sepalen zijn geli jk van kleur, grootte en vorm en vaak zijn ze bruin gestreept. Meestal zijn de twee petalen korter dan de sepalen en hebben ook een andere kleur. Ze staan naar de zuil toe gebogen.