Predicting Suitable Habitat of the Chinese Monal (Lophophorus Lhuysii) Using Ecological Niche Modeling in the Qionglai Mountains, China
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Establish an Environmentally Sustainable Giant Panda National Park in the Qinling Mountains
Science of the Total Environment 668 (2019) 979–987 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Establish an environmentally sustainable Giant Panda National Park in the Qinling Mountains Yan Zhao a,b,Yi-pingChena,c,⁎, Aaron M. Ellison d,Wan-gangLiua,DongChena,b a SKLLQG, Institute of Earth Environment, Chinese Academy of Sciences, Xi'an 710075, China b University of Chinese Academy of Sciences, Beijing 10049, China c CAS Center for Excellence in Quaternary Science and Global Change, Xi'an 710061, China d Harvard University, Harvard Forest, Petersham, MA, USA HIGHLIGHTS GRAPHICAL ABSTRACT • Heavy metals contents increased from core, buffer to environmental areas in Qinling. • Heavy metal distribution was correlated with altitude and latitude in Qinling. • Minimizing heavy metals emission is a long-term task for panda conservation. • Expanding core area and adherence to the basic principle of functional areas • Establishing pollutants monitoring and staple bamboo protection article info abstract Article history: The giant panda (Ailuropoda melanoleuca) is one of the most endangered animals in the world and is recognized Received 9 January 2019 worldwide as a symbol for conservation. The Qinling subspecies of giant panda (Ailuropoda melanoleuca Received in revised form 5 March 2019 qinlingensis) is highly endangered; fewer than 350 individuals still inhabit the Qinling Mountains. Last year, Accepted 5 March 2019 China announced the establishment of the first Giant Panda National Park (GPNP) with a goal of restoring and Available online 06 March 2019 connecting fragmented habitats; the proposal ignored the environmental pollution caused by economic develop- Editor: Damia Barcelo ment in panda habitats. -

ISG Capital Management, Ltd
ISG Capital Management Ltd 盛集投资 ISG Capital Management Ltd 14 Wall Street, 20th Floor, NY, NY USA 10005 1366 West Nanjing Road, 15th Fl, Plaza 66-II, Shanghai, 200040, China www.isgfn.com _________________________________________________________________________________________________ SCHOOL RECONSTRUCTION PROJECT – SICHUAN, CHINA Dear Friends, The magnitude 8 earthquake that hit southwestern China's Sichuan Province on May 12th, 2008 destroyed thousands of buildings, roads, schools and hospitals and claimed over 50,000 lives. In just 12 seconds, more than 170 towns, including those in the proximity of Chengdu City, were either destroyed or badly damaged. More than 45 million people were affected by the earthquake—the worst natural disaster to hit China in 30 years. ISG Capital Management, Ltd. (―ISG‖) is a private equity real estate investment firm based in Shanghai, China and in New York. Our team is made up of highly experienced real estate professionals. We at ISG are especially saddened by the tragedy due to our longstanding relationship with the disaster area. Many of our staff are either from that region or have close friends and relatives still living there. Some staff members have been working on development projects around Chengdu and elsewhere in Sichuan for years. Our founder, Li Li, began her career as a high school teacher in the 1980s and is from a family of educators whose hometown is Chengdu. Our close ties to the stricken region and our real estate expertise have led us to the conclusion that the best long- term contribution that ISG can make to help the people affected by this tragedy is to rebuild a school for the children of Dayi County. -

1 This Research Project Has Been Approved by The
Adaptability Evaluation of Human Settlements in Chengdu Based on 3S Technology Wende Chen Chengdu University of Technology kun zhu ( [email protected] ) Chengdu University of Technology https://orcid.org/0000-0003-2871-4155 QUN WU Chengdu University of Technology Yankun CAI Chengdu University of Technology Yutian LU Chengdu University of Technology jun Wei Chengdu University of Technology Research Article Keywords: Human settlement, Evaluation, 3s technology, Spatial differentiation, Chengdu city Posted Date: February 22nd, 2021 DOI: https://doi.org/10.21203/rs.3.rs-207391/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License 1 Ethical Approval: 2 This research project has been approved by the Ethics Committee of Chengdu University of Technology. 3 Consent to Participate: 4 Written informed consent for publication was obtained from all participants. 5 Consent to Publish: 6 Author confirms: The article described has not been published before; Not considering publishing elsewhere; Its 7 publication has been approved by all co-authors; Its publication has been approved (acquiesced or publicly approved) by 8 the responsible authority of the institution where it works. The author agrees to publish in the following journals, and 9 agrees to publish articles in the corresponding English journals of Environmental Science and Pollution Research. If the 10 article is accepted for publication, the copyright of English articles will be transferred to Environmental Science and 11 Pollution Research. The author declares that his contribution is original, and that he has full rights to receive this grant. 12 The author requests and assumes responsibility for publishing this material on behalf of any and all co-authors. -

GIS Assessment of the Status of Protected Areas in East Asia
CIS Assessment of the Status of Protected Areas in East Asia Compiled and edited by J. MacKinnon, Xie Yan, 1. Lysenko, S. Chape, I. May and C. Brown March 2005 IUCN V 9> m The World Conservation Union UNEP WCMC Digitized by the Internet Archive in 20/10 with funding from UNEP-WCMC, Cambridge http://www.archive.org/details/gisassessmentofs05mack GIS Assessment of the Status of Protected Areas in East Asia Compiled and edited by J. MacKinnon, Xie Yan, I. Lysenko, S. Chape, I. May and C. Brown March 2005 UNEP-WCMC IUCN - The World Conservation Union The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of UNEP, UNEP-WCMC, and IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. UNEP-WCMC or its collaborators have obtained base data from documented sources believed to be reliable and made all reasonable efforts to ensure the accuracy of the data. UNEP-WCMC does not warrant the accuracy or reliability of the base data and excludes all conditions, warranties, undertakings and terms express or implied whether by statute, common law, trade usage, course of dealings or otherwise (including the fitness of the data for its intended use) to the fullest extent permitted by law. The views expressed in this publication do not necessarily reflect those of UNEP, UNEP-WCMC, and IUCN. Produced by: UNEP World Conservation Monitoring Centre and IUCN, Gland, Switzerland and Cambridge, UK Cffti IUCN UNEP WCMC The World Conservation Union Copyright: © 2005 UNEP World Conservation Monitoring Centre Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. -

Predicting Global Population Connectivity and Targeting Conservation Action for Snow Leopard Across Its Range
Ecography 39: 419–426, 2016 doi: 10.1111/ecog.01691 © 2015 e Authors. Ecography © 2015 Nordic Society Oikos Subject Editor: Bethany Bradley. Editor-in-Chief: Miguel Araújo. Accepted 27 April 2015 Predicting global population connectivity and targeting conservation action for snow leopard across its range Philip Riordan, Samuel A. Cushman, David Mallon, Kun Shi and Joelene Hughes P. Riordan ([email protected]) and J. Hughes, Dept of Zoology, Univ. of Oxford, South Parks Road, Oxford, OX1 3PS, UK. – S. A. Cushman, US Forest Service, Rocky Mountain Research Station, 800 E Beckwith, Missoula, MT 59801, USA. – D. Mallon, Division of Biology and Conservation Ecology, School of Science and the Environment, Manchester Metropolitan Univ., Manchester, M1 5GD, UK. – K. Shi and PR, Wildlife Inst., College of Nature Conservation, Beijing Forestry Univ., 35, Tsinghua-East Road, Beijing 100083, China. Movements of individuals within and among populations help to maintain genetic variability and population viability. erefore, understanding landscape connectivity is vital for effective species conservation. e snow leopard is endemic to mountainous areas of central Asia and occurs within 12 countries. We assess potential connectivity across the species’ range to highlight corridors for dispersal and genetic flow between populations, prioritizing research and conservation action for this wide-ranging, endangered top-predator. We used resistant kernel modeling to assess snow leopard population connectivity across its global range. We developed an expert-based resistance surface that predicted cost of movement as functions of topographical complexity and land cover. e distribution of individuals was simulated as a uniform density of points throughout the currently accepted global range. -

Zipingpuresettle.Pdf
The overall condition of resettlement for Zipingpu hydraulic project The Zipingpu hydraulic project involves resettlement of 33,000 people in a total of 5 townships, which are located in Dujiangyan city (under the administration of Chengdu municipality of Sichuan Province) and Wenchuan county of Aba Tibetan Ethinicty Autonomous Prefecture, and 29 county-owned corporations. Among them, 11,000 came from Dujiangyan city and the rest from Wenchuan. The affected people in Dujiangyan city are mainly located in Chaguan village of Longxi township, Maxi township and its Qingyuan village, Ziping village and Dujiang village of Zipingpu township. These affected communities are relocated to Nanhai, Quanhong, Kuangjia, Jijia and Ximin villages in Jiahongxiang township of Dujiangyan. The affected people in Wenchuan county are mainly located in Xuankou township and its Zhao-er-ba, Shuitianping and Xuaokou villages, Baihua township and its Shenyinsi, Baihuatan and Youzhan villages, and Yingxiu township and its Baiyan village. About 10,000 people of the Wenchuan’s affected people were relocated to Wenjiang, Chongzhou, Qionglai, Longquan, Xinjin, Shuangliu, Xindu and Pengzhou county or district of Chengdu municipality. The major issues of Zipingpu dam resettlement The affected communities and individuals as the land and property owners are not entitled to assessing the standard of compensation The Sichuan Institute of Water Resources and Surveying, which is closely related to the Zipingpu Hydropower Company, conducted the assessment of the compensation standard. The government released the results to the public. The whole process was conducted in the black box. The related documents and policies did not go through the consultation and hearing processes before the resettlement started. -

Asian Alpine E-News Issue No.19
ASIAN ALPINE E–NEWS Issue No.19, February 2018 CONTENTS 1. Mountains and Mountaineering in Art and Literature Tony Astill F.R.G.S. Les Alpes Livres Page 2 2. Yoshio Ogata’s Photo Collection “Mountains of Sikkim” from new book published by the Himalayan Association of Japan Photo copyrights: Yoshio Ogata (Supplements with Tom Nakamura’s Aerial Views) Page 3 ~23 3. News and Photo Album Queen Valley of Beauties & Mt. Siguniang, Qionglai Mountains Hengduan Mountains in West Sichuan Kenzo Okawa Page24~46 1 Mountains and Mountaineering in Art and Literature Tony Astill F.R.G.S. Les Alpes Livres Tony Astill has been selling old and rare mountaineering books for more than 40 years and also fine mountain paintings for 25 years. A very large selection can be seen on his websites www.mountaineeringbooks.org and www.mountainpaintings.org His book ‘Mount Everest : The Reconnaissance 1935’ won the James Monroe Thorington Award for best book Mountaineering History at the Banff Mountain Festival 2006. Tony will be very pleased to hear from all those who would like to contact him [email protected] T. Howard Somervell, [1890-1975] Lhotse, Everest and Makalu from Singalila, Sandakphu. 1943 watercolour and gouache over pencil, on his usual Kraft brown paper. signed with date twice, lower left. 29 x 36 cms. Yoshio Ogata’s Photo Collection “Mountains of Sikkim” from new book published by the Himalayan Association of Japan Photo copyrights: Yoshio Ogata (Supplements with Tom Nakamura’s Aerial Views over the Himalaya) Kangchenjunga northeast ridge, lower -
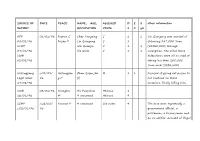
SOURCE of DATE PLACE NAME, AGE, ALLEGED D E 2 Other Information REPORT OCCUPATION CRIME S X Yrs
SOURCE OF DATE PLACE NAME, AGE, ALLEGED D E 2 other information REPORT OCCUPATION CRIME S X yrs AFP 01/01/96 Fuzhou C Chen Yongxing C 1 1 Lin Qiangong was accused of 08/01/96 Fujian P Lin Qiangong C 1 obtaining 247,000 Yuan SCMP Wei Quanjin C 1 1 (US$30,000) through 09/01/96 Xie Qixin C 1 1 corruption. The other three SWB defendants were all accused of 02/02/96 taking less than 200,000 Yuan each (US$2,500) Heilongjiang c.01/01/ Heilongjian Zhan Qiqun,36 M 1 1 Accused of giving rat poison to Legal News 96 g P (f) her husband on three 17/08/96 occasions, finally killing him. SWB 02/01/96 Shanghai Hu Yuanchun Heinous 1 16/01/96 M 9 unnamed Heinous 9 SCMP c.02/01/ Yunnan P 4 unnamed See notes 4 The four were reportedly a c.02/01/96 96 government official, a policeman, a businessman and an ex-soldier. Accused of illegal elephant hunting. SCMP c.02/01/ Shijiazhuan 13 unnamed M, Rob 1 1 c.02/01/96 96 g 3 3 Hebei P FBIS 05/01/96 Shenzhen C 1 unnamed Rob 1 1 Accused of train robbery. 11/01/96 Guangdong SWB P 16/01/96 SWB 05/01/96 Foshan C Lin Zhentao E 1 Accused of embezzling 7.82 02/02/96 Guangdong million Yuan (US$939,759). FBIS P 11/01/96 Shanghai c.08/01/ Shanghai Gao Qiming R 1 Some of these sentences were Legal News 96 M Hu Yuanqing H 1 reportedly suspended for two 08/01/96 Lu Hongbao M 1 years; the report does not SWB Yan Changbing M 1 indicate the names of the 02/02/96 Zhang Xiaodong T 1 prisoners. -

THE ROOTED STATE: PLANTS and POWER in the MAKING of MODERN CHINA's XIKANG PROVINCE by MARK E. FRANK DISSERTATION Submitted In
THE ROOTED STATE: PLANTS AND POWER IN THE MAKING OF MODERN CHINA’S XIKANG PROVINCE BY MARK E. FRANK DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in East Asian Languages and Cultures in the Graduate College of the University of Illinois at Urbana-Champaign, 2020 Urbana, Illinois Doctoral Committee: Associate Professor Dan Shao, Chair Associate Professor Robert Morrissey Assistant Professor Roderick Wilson Associate Professor Laura Hostetler, University of Illinois Chicago Abstract This dissertation takes the relationship between agricultural plants and power as its primary lens on the history of Chinese state-building in the Kham region of eastern Tibet during the early twentieth century. Farming was central to the way nationalist discourse constructed the imagined community of the Chinese nation, and it was simultaneously a material practice by which settlers reconfigured the biotic community of soils, plants, animals, and human beings along the frontier. This dissertation shows that Kham’s turbulent absorption into the Chinese nation-state was shaped by a perpetual feedback loop between the Han political imagination and the grounded experiences of soldiers and settlers with the ecology of eastern Tibet. Neither expressions of state power nor of indigenous resistance to the state operated neatly within the human landscape. Instead, the rongku—or “flourishing and withering”—of the state was the product of an ecosystem. This study chronicles Chinese state-building in Kham from Zhao Erfeng’s conquest of the region that began in 1905 until the arrival of the People’s Liberation Army in 1950. Qing officials hatched a plan to convert Kham into a new “Xikang Province” in the last years of the empire, and officials in the Republic of China finally realized that goal in 1939. -

Connecting Sichuan
Connecting Sichuan A landmark partnership to revitalize communities by transforming healthcare, education, and the workforce Rebuilding Better, Together The people of Sichuan suffered great losses when a Social Impact at a Glance massive earthquake devastated their province in May 2008. In addition to significant loss of life, the earthquake Indicator Description Metric as of June 2011 destroyed many schools and hospitals located in rural, Community Counties within Sichuan 8 out of 10 of the hardest hard to reach areas. Cisco, the Cisco Foundation, and our investment benefiting from the program hit counties employees immediately responded by donating more Economic Program social investment US$50 million than US$2.6 million (about RMB 16.8 million) in grants and investment relief funds. But a longer-term response was needed to Building capacity Commercial, NGO, and 40 partners* restore and revitalize the region. Cisco and the Chinese through partners government partners contributing to the program government saw an opportunity for renewal in the midst of the Sichuan destruction—an opportunity to rebuild better, 21st century ICT Number of network-enabled 193 infrastructure healthcare and education together. institutions That vision for a better future resulted in the creation of 21st century skills Investment in professional +9,900 healthcare and development development and ICT skills education professionals a unique public-private partnership, a three-year Cisco trained corporate social responsibility program called Connecting Sichuan. * Excludes healthcare organizations and educational institutions Connecting Sichuan was designed to systematically transform healthcare, education, and the workforce About Sichuan in the province through the use of information and Long known as China’s Province of Abundance, communications technology (ICT). -

Giant Panda Sp.Ac.01
Giant Panda IUCN Status Category: Endangered Ailuropoda melanoleuca (David, 1869) CITES Appendix: I INTRODUCTION Giant pandas are robust members of the bear family with a distinctive black and white coat. Their head and body length is 120 to 190 cm, and adults weigh 85 to 125 kg. Specialized features include broad, flat molars modified for crushing, and an enlarged wristbone functioning as an opposable thumb — both adaptations for eating bamboo. The giant panda’s diet consists almost entirely of the leaves, stems and shoots of various bamboo species; although they occasionally eat meat. A giant panda may consume 12 to 18 kg of bamboo a day to meet its energy requirements. Giant pandas inhabit the bamboo forest zone between 1,200 m and 3,400 m. Formerly they were found in riverine valleys at lower elevations, but these areas are now settled by humans. Giant pandas are generally solitary, each adult having a well-defined home range. A male’s home range overlaps with those of several females. Although encounters are rare outside the brief mating season, pandas communicate fairly often mostly through vocalization and scent marking. Giant pandas reach sexual maturity between 4.5 and 7.5 years. After a gestation period of about five months, females give birth to a single young or sometimes twins. Wild giant pandas bear a cub every two years or more. Newborns are tiny, weighing only 100 to 160 grams. Cubs start eating bamboo at about one year of age, but remain with their mother until she conceives again, usually when the cub is about 18 months old. -

Groundbreaking for Swiss Community Project in Chongzhou: Longxing Nursery School
GROUNDBREAKING FOR SWISS COMMUNITY PROJECT IN CHONGZHOU: LONGXING NURSERY SCHOOL (May 20, 2009, Chongzhou City, Sichuan Province) The Swiss Community Reconstruction Project in Sichuan broke ground for the central Kindergarten in Longxing town of Chongzhou city, one of the areas affected by the earthquake in 2008. The groundbreaking ceremony took place at the new site for the Longxing Nursery School. It was attended by local government officials and other attending sponsors and partners of the Swiss Community in China, especially Mr. Blaise Godet, Ambassador of Switzerland in China, Mr. Christian Guertler, Chairman of SwissCham China, Mr. Stephan Titze and Mr. Felix Sutter, CoPresidents of the Steering Committee of the project and Mr. Daniel Heusser, Architect and Director of the project. Mr. Blaise Godet, Ambassador of Switzerland in China, officials from Chongzhou City and representatives of the donors shoveled the first soil for the Longxing Nursery School. This historic action will be recorded in the annuals of the kindergarten as the official start of the construction phase for Swiss Community Reconstruction Project. This reconstruction project financed mainly by the Swiss business community in China and Switzerland was initiated a few days after the earthquake by SwissCham Shanghai, co sponsored by SwissCham Beijing and has gained the patronage of the Swiss Embassy, the Consulates General in Shanghai and Guangzhou, the Swiss Club in Shanghai and the Swiss Society in Beijing. Up to date, the Swiss Community has raised 6,250,000 RMB in financial donations and multiple nonfinancial support commitments for the supply and transportation of materials. In addition, many volunteer hours have been spent and committed for the realization of the project.