Fostamatinib
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tavalisse™ (Fostamatinib) (Oral) Document Number: IC-0361 Last Review Date: 02/02/2021 Date of Origin: 06/01/2018 Dates Reviewed: 06/2018, 02/2019, 02/2020, 02/2021
Tavalisse™ (fostamatinib) (Oral) Document Number: IC-0361 Last Review Date: 02/02/2021 Date of Origin: 06/01/2018 Dates Reviewed: 06/2018, 02/2019, 02/2020, 02/2021 I. Length of Authorization Coverage is provided for six months and may be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: 100 mg tablets – 2 tablets per day 150 mg tablets – 2 tablets per day B. Max Units (per dose and over time) [HCPCS Unit]: 300 mg daily III. Initial Approval Criteria 1,2 Coverage is provided in the following conditions: Patient is at least 18 years of age; AND Universal Criteria 1 Patient is not receiving a thrombopoietin receptor agonist or mimetic (e.g., romiplostim, eltrombopag, lusutrombopag, avatrombopag, etc.); AND Laboratory values are current (i.e., drawn within the previous 28 days); AND Fostamatinib is not being used to attempt to normalize platelet count; AND Patient will avoid concomitant therapy with any of the following: o Coadministration with strong CYP3A4 inhibitors (e.g., clarithromycin, itraconazole, ketoconazole, etc.), or if therapy is unavoidable, the patient will be monitored closely for adverse reaction and/or dose modifications will be implemented; AND o Coadministration with strong CYP3A inducers (e.g., rifampin, carbamazepine, St. John’s Wort, etc.); AND Chronic Immune Thrombocytopenia (ITP) † 1-5 Patient has had persistent/chronic ITP for at least 3 months; AND Proprietary & Confidential © 2021 Magellan Health, Inc. Patient has previously failed any of the following treatments for ITP: Patient has failed previous therapy with corticosteroids (i.e., patient had no response to at least a 3-month trial or is corticosteroid-dependent); OR Patient has failed previous therapy with immunoglobulins; OR Patient has had a splenectomy; OR Patient has failed previous therapy with a thrombopoietin receptor agonist; AND The patient is at increased risk for bleeding as indicated by platelet count of less than 30 × 109/L (30,000/mm³) † FDA Approved Indication(s) IV. -

Blood Modifier Agents Policy #: Rx.01.208
Pharmacy Policy Bulletin Title: Blood Modifier Agents Policy #: Rx.01.208 Application of pharmacy policy is determined by benefits and contracts. Benefits may vary based on product line, group, or contract. Some medications may be subject to precertification, age, quantity, or formulary restrictions (ie limits on non-preferred drugs). Individual member benefits must be verified. This pharmacy policy document describes the status of pharmaceutical information and/or technology at the time the document was developed. Since that time, new information relating to drug efficacy, interactions, contraindications, dosage, administration routes, safety, or FDA approval may have changed. This Pharmacy Policy will be regularly updated as scientific and medical literature becomes available. This information may include new FDA-approved indications, withdrawals, or other FDA alerts. This type of information is relevant not only when considering whether this policy should be updated, but also when applying it to current requests for coverage. Members are advised to use participating pharmacies in order to receive the highest level of benefits. Intent: The intent of this policy is to communicate the medical necessity criteria for eltrombopag olamine (Promacta®), fostamatinib disodium (Tavalisse™), avatrombopag (Doptelet®), lusutrombopag (Mulpleta®) as provided under the member's prescription drug benefit. Description: Idiopathic thrombocytopenia purpura (ITP): ITP is an immune disorder in which the blood doesn't clot normally. ITP can cause excessive bruising and bleeding and can be characterized as an unusually low level of platelets, or thrombocytes, in the blood results in ITP. Thrombocytopenia in patients with hepatitis C: Thrombocytopenia can occur in patients with chronic hepatitis C virus (HCV) infection. -

Farmaatsia- Terminoloogia Teine, Täiendatud Trükk
Farmaatsia- terminoloogia Teine, täiendatud trükk Graanulid Suspensioon Lahus Emulsioon Pillid Pulber Salv Kreem Aerosool Plaaster Sprei Pastill Tampoon Oblaat Emulsioon Kontsentraat Silmageel Tablett Haavapulk Ninatilgad Kapsel Lakukivi Inhalaator Farmaatsia- terminoloogia Teine, täiendatud trükk Tartu 2019 Koostajad: Toivo Hinrikus, Karin Kogermann, Ott Laius, Signe Leito, Ain Raal, Andres Soosaar, Triin Teppor, Daisy Volmer Keeletoimetaja: Tiina Kuusk Kirjastanud: Ravimiamet Nooruse 1, 50411 Tartu Telefon: +372 737 4140 Faks: +372 737 4142 E-post: [email protected] Esimene trükk 2010 Teine, täiendatud trükk 2019 Raamat on leitav Ravimiameti veebilehelt: www.ravimiamet.ee/farmaatsiaterminoloogia Väljaande refereerimisel või tsiteerimisel palume viidata allikale. ISBN 978-9949-9697-3-9 Sisukord Farmaatsiaterminoloogia Eestis..........................................................................................5 Üldised farmaatsiaalased terminid ...................................................................................10 Euroopa farmakopöa ......................................................................................................... 21 Euroopa farmakopöa sõnastik ..........................................................................................24 Standardterminid ..............................................................................................................29 Ravimvormid .....................................................................................................................29 -

New Drug Evaluation Monograph Template
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-2596 Drug Class Review: Thrombocytopenia Date of Review: January 2019 End Date of Literature Search: 11/05/2018 Purpose for Class Review: Treatments for thrombocytopenia are being reviewed for the first time, prompted by the recent approval of three new drugs; avatrombopag (Doptelet®), fostamatinib (Tavalisse™) and lusutrombopag (Mulpleta®). Research Questions: 1. What is the evidence for efficacy and harms of thrombocytopenia treatments (avatrombopag, eltrombopag, lusutrombopag, fostamatinib, and romiplostim)? 2. Is there any comparative evidence for therapies for thrombocytopenia pertaining to important outcomes such as mortality, bleeding rates, and platelet transfusions? 3. Is there any comparative evidence based on the harms outcomes of thrombocytopenia treatments? 4. Are there subpopulations of patients for which specific thrombocytopenia therapies may be more effective or associated with less harm? Conclusions: Three guidelines, six randomized clinical trials and five high-quality systematic reviews and meta-analyses met inclusion criteria for this review. There was insufficient direct comparative evidence between different therapies to treat thrombocytopenia. A majority of trials were small and of short duration. Guidelines recommend corticosteroids and intravenous immunoglobulin (IVIg) as first-line therapy for most adults with idiopathic thrombocytopenia (ITP). Thrombopoietin receptor agonists (TPOs) and the tyrosine kinase inhibitor, fostamatinib, are recommended as second-line treatments after failure of at least one other treatment.1–3 Avatrombopag and lusutrombopag are only approved for short-term use before procedures in patients with chronic liver failure. -

Nplate® (Romiplostim)
Nplate® (romiplostim) (Subcutaneous) Document Number: IC-0089 Last Review Date: 02/02/2021 Date of Origin: 01/01/2012 Dates Reviewed: 12/2011, 02/2013, 02/2014, 12/2014, 10/2015, 09/2016, 12/2016, 03/2017, 06/2017, 12/2017, 03/2018, 06/2018, 10/2018, 01/2019, 12/2019, 02/2020, 02/2021 I. Length of Authorization Coverage will be provided for 3 months and may be renewed, unless otherwise specified. • Coverage for use to treat Hematopoietic Syndrome of Acute Radiation Syndrome (HSARS) cannot be renewed. II. Dosing Limits A. Quantity Limit (max daily dose) [NDC Unit]: − Nplate 125 mcg SDV for injection: 4 vials per 28 days − Nplate 250 mcg SDV for injection: 20 vials per 28 days − Nplate 500 mcg SDV for injection: 12 vials per 28 days B. Max Units (per dose and over time) [HCPCS Unit]: • ITP: 125 billable units weekly • MDS: 100 billable units weekly • HSARS: 125 billable units x 1 dose III. Initial Approval Criteria 1,3,13,14 Coverage is provided in the following conditions: Universal Criteria 1 • Patient is not on any other thrombopoietin receptor agonist or mimetic (e.g., lusutrombopag, eltrombopag, avatrombopag, etc.) or fostamatinib; AND • Must not be used in an attempt to normalize platelet counts; AND • Laboratory value for platelet count is current (i.e., drawn within the previous 28 days); AND Immune (idiopathic) thrombocytopenia (ITP) † Ф 1,412 Proprietary & Confidential © 2021 Magellan Health, Inc. • The patient is at increased risk for bleeding as indicated by platelet count less than 30 × 109/L (30,000/mm³); AND o Patient has acute ITP; AND . -

Perioperative Medication Management - Adult/Pediatric - Inpatient/Ambulatory Clinical Practice Guideline
Effective 6/11/2020. Contact [email protected] for previous versions. Perioperative Medication Management - Adult/Pediatric - Inpatient/Ambulatory Clinical Practice Guideline Note: Active Table of Contents – Click to follow link INTRODUCTION........................................................................................................................... 3 SCOPE....................................................................................................................................... 3 DEFINITIONS .............................................................................................................................. 3 RECOMMENDATIONS ................................................................................................................... 4 METHODOLOGY .........................................................................................................................28 COLLATERAL TOOLS & RESOURCES..................................................................................................31 APPENDIX A: PERIOPERATIVE MEDICATION MANAGEMENT .................................................................32 APPENDIX B: TREATMENT ALGORITHM FOR THE TIMING OF ELECTIVE NONCARDIAC SURGERY IN PATIENTS WITH CORONARY STENTS .....................................................................................................................58 APPENDIX C: METHYLENE BLUE AND SEROTONIN SYNDROME ...............................................................59 APPENDIX D: AMINOLEVULINIC ACID AND PHOTOTOXICITY -

May 2017 Monitoring International Trends
Monitoring International Trends posted April-May 2017 The NBA monitors international developments that may influence the management of blood and blood products in Australia. Our focus is on: • Potential new product developments and applications; • Global regulatory and blood practice trends; • Events that may have an impact on global supply, demand and pricing, such as changes in company structure, capacity, organisation and ownership; and • Other emerging risks that could potentially put financial or other pressures on the Australian sector. A selection of recent matters of interest appears below. Highlights include: Haemophilia Treatment • Pfizer has made a deal with Sangamo on a new gene therapy for haemophilia A. • Dimension Therapeutics discontinued its development of an investigational gene therapy for haemophilia B • uniQure’s investigational gene therapy for patients with haemophilia B has been awarded PRIME status by European regulators, setting the treatment on a faster path to possible approval. • Australia’s Therapeutic Goods Administration (TGA) approved CSL Behring’s Afstyla, a recombinant single chain coagulation factor VIII, for patients with haemophilia A. • Aptevo presented data on Ixinity, its recombinant factor IX product. • Octapharma USA presented study on the development of inhibitors in haemophilia patients, and on the need for frequent venous access for FVIII injection. • The European Union (EU) Committee for Medicinal Products for Human Use (CHMP) recommended marketing authorisation for Novo Nordisk’s long-acting Factor IX, nonacog beta pegol (Refixia). Patient Blood Management and Patient Safety • The US FDA, accepting that near the end of their shelf life platelets are at a greater risk of bacterial growth, has suggested that platelets need to be retested. -
Committee for Medicinal Products for Human Use (CHMP) Agenda for the Meeting on 14-17 October 2019 Chair: Harald Enzmann – Vice-Chair: Bruno Sepodes
14 October 2019 EMA/CHMP/556731/2019 Inspections, Human Medicines Pharmacovigilance and Committees Division Committee for medicinal products for human use (CHMP) Agenda for the meeting on 14-17 October 2019 Chair: Harald Enzmann – Vice-Chair: Bruno Sepodes 14 October 2019, 13:00 – 19:30, room 1C 15 October 2019, 08:30 – 19:30, room 1C 16 October 2019, 08:30 – 19:30, room 1C 17 October 2019, 08:30 – 16:00, room 1C Health and safety information In accordance with the Agency’s health and safety policy, delegates are to be briefed on health, safety and emergency information and procedures prior to the start of the meeting. Disclaimers Some of the information contained in this agenda is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scopes listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also vary during the course of the review. Additional details on some of these procedures will be published in the CHMP meeting highlights once the procedures are finalised and start of referrals will also be available. Of note, this agenda is a working document primarily designed for CHMP members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the agenda cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on-going procedures for which a final decision has not yet been adopted. -
Tenncare Attestation List September 1, 2021
TennCare Attestation List September 1, 2021 Medications on this list can be approved for patients currently at their monthly prescription limit (greater than 5 prescriptions or greater than 2 brand medications), who are at a high risk for adverse health consequences and could be hospitalized, institutionalized, or die within the next 90 days without the requested drug(s). This list is independent of the preferred drug list (PDL) and other criteria. All drugs on this list are still subject to existing TennCare edits, including non- preferred status on the PDL, clinical criteria, step therapy criteria, and quantity limits.* ANTI-INFECTIVES Aminoglycosides • amikacin (Amikin, Arikayce) • neomycin (Neo-Fradin) • gentamicin (Garamycin) • paromomycin • kanamycin • streptomycin Cephalosporins, 1st Generation • cefadroxil (Duricef) • cephalexin (Keflex) • cefazolin (Ancef) Cephalosporins, 2nd Generation • cefaclor (Ceclor) • cefprozil (Cefzil) • cefoxitin (Mefoxin) • cefuroxime (Ceftin, Zinacef) Cephalosporins, 3rd Generation • cefdinir • ceftazidime (Fortaz, Tazicef) • cefditoren (Spectracef) • ceftibuten (Cedax) • cefixime (Suprax) • ceftizoxime (Cefizox) • cefotaxime (Claforan) • ceftriaxone (Rocephin) • cefpodoxime (Vantin) Cephalosporins, 4th Generation • cefepime (Maxipime) Ketolides • telithromycin (Ketek) Lincosamides • clindamycin (Cleocin) • lincomycin (Lincocin) Macrolides • azithromycin (Zithromax) • erythromycin (E.E.S., Eryc, Ery-Tab, EryPed) • clarithromycin (Biaxin, Biaxin XL) • erythromycin/sulfisoxazole (Eryzole, Pediazole) -

Aralast NP™, Glassia™, Prolastin®-C
Wednesday, May 10, 2017 4 p.m. Oklahoma Health Care Authority 4345 N. Lincoln Blvd. Oklahoma City, OK 73105 The University of Oklahoma Health Sciences Center COLLEGE OF PHARMACY PHARMACY MANAGEMENT CONSULTANTS MEMORANDUM TO: Drug Utilization Review Board Members FROM: Bethany Holderread, Pharm.D. SUBJECT: Packet Contents for Board Meeting – May 10, 2017 DATE: May 1, 2017 NOTE: The DUR Board will meet at 4:00 p.m. The meeting will be held at 4345 N Lincoln Blvd. Enclosed are the following items related to the May meeting. Material is arranged in order of the agenda. Call to Order Public Comment Forum Action Item – Approval of DUR Board Meeting Minutes – Appendix A Update on Medication Coverage Authorization Unit/Spring Pipeline Update – Appendix B Action Item – Vote to Prior Authorize Fosamax® (Alendronate 40mg Tablets) – Appendix C Action Item – Vote to Prior Authorize Byvalson™ (Nebivolol/Valsartan) and Qbrelis™ (Lisinopril Oral Solution) – Appendix D Action Item – Vote to Prior Authorize Giazo® (Balsalazide Disodium Tablets) – Appendix E Action Item – Vote to Prior Authorize Invokamet® XR (Canagliflozin/Metformin Extended-Release), Jentadueto® XR (Linagliptin/Metformin Extended-Release), Adlyxin® (Lixisenatide), Xultophy® 100/3.6 (Insulin Degludec/Liraglutide), Soliqua™ 100/33 (Insulin Glargine/Lixisenatide), Synjardy® XR (Empagliflozin/Metformin Extended-Release), and Qtern® (Dapagliflozin/Saxagliptin) – Appendix F Annual Review of Lung Cancer Medications and 30-Day Notice to Prior Authorize Tarceva® (Erlotinib), Gilotrif® (Afatinib), -

Lääkeaineiden Yleisnimet (INN-Nimet) 31.12.2019
Lääkealan turvallisuus- ja kehittämiskeskus Säkerhets- och utvecklingscentret för läkemedelsområdet Finnish Medicines Agency Lääkeaineiden yleisnimet (INN-nimet) 31.12. -
Compatibility Mode
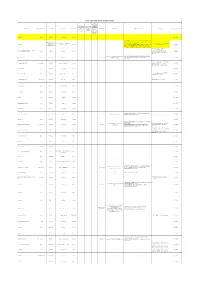
YURT DIŞI AKTİF ETKİN MADDE LİSTESİ Acil Durumlarda TİTCK Yazılı Kullanımı Sadece Toplu Onayı Olmadan İçin TİTCK Teminine İzin Etkin Madde Farmasötik form ATC kodu ATC Adı Reçete Türü İthal Tarafından ICD10 Kodu ICD10 Adı Kullanım Şartları Açıklama Listeye Eklenme Tarihi Verilen Edilemeyecek Hastanelere İlaçlar İlaçlar Toplu Temin İzni Verilen İlaçlar Erdafitinib tablet L01EX16 erdafitinib Normal 1 24.08.2020 ORTA/İLERİ EVRE COVID-19 TANISI ALMIŞ HASTALAR, REMDESİVİR KULLANIMI İÇİN AŞAĞIDAKİ KRİTERLERİN DSÖ tarafından ATC DSÖ tarafından ATC kodu henüz TÜMÜNÜ KARŞILAMALIDIR: Tedariği Halk Sağlığı Genel Müdürlüğü Remdesivir vial kodu henüz Normal 25.05.2020 belirlenmemiş ilaç 1) 18 YAŞ VE ÜZERİ ERKEK VEYA KADIN (GEBE tarafından sağlanacaktır. belirlenmemiş ilaç HASTALAR VE EMZİREN HASTALAR HARİÇ) OLMASI 2) TEMAS ÖYKÜSÜ OLAN VEYA UYGUN KLİNİK YAKINMALAR EŞLİĞİNDE PCR VE/VEYA KABUL EDİLEN Son 1 yıl içinde temin edilmemesi nedeniyle "Pasif Etkin Maddeler (RS)-2,3-bis(sulphanyl)propane-1-sulphonic capsul V03AB antidotes Normal 1 Listesi"ndeyken, USHAŞ'ın talebiyle 9.03.2020 acid, sodium salt-monohydrate yeniden "Aktif Etkin Madde Listesi" ne alınmıştır. 1-KONJENİTAL MYASTENİA TEDAVİSİNDE KULLANILIR. Konjenital ve gelişimsel miyastenia;Eaton- 3,4- diaminopyridine tablet N07XX05 amifampridine Normal G70.2;G73.1 2-EATON LAMBERT SENDROMU TEDAVİSİNDE 18.07.2014 Lambert sendromu KULLANILIR. **"Sadece Toplu Teminine İzin Verilen İlaçlar" kategorisinden çıkartılarak "Acil 5-Aminolevulinic acid oral solution L01XD04 aminolevulinic acid Normal 1 Durumlarda Kullanımı İçin TİTCK 2.04.2018 Tarafından Hastanelere Toplu Temin İzni Verilen İlaçlar" kategorisine alınmıştır. 8-Methoxypsoralen vial D05BA02 methoxsalen Normal 1 18.07.2016 12.09.2016 tarihinden itibaren TİTCK Abacavir, lamivudine tablet J05AR02 lamivudine and abacavir Normal 1 18.07.2014 yazılı onay şartı eklenmiştir.