Prerequisites in Order to Be Successful in AP Chemistry, Students Must
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Relationship of PSAT/NMSQT Scores and AP Examination Grades
Research Notes Office of Research and Development RN-02, November 1997 The Relationship of PSAT/NMSQT Scores and AP® Examination Grades he PSAT/NMSQT, which measures devel- Recent analyses have shown that student per- oped verbal and quantitative reasoning, as formance on the PSAT/NMSQT can be useful in Twell as writing skills generally associated identifying additional students who may be suc- with academic achievement in college, is adminis- cessful in AP courses. PSAT/NMSQT scores can tered each October to nearly two million students, identify students who may not have been initially the vast majority of whom are high school juniors considered for an AP course through teacher or and sophomores. PSAT/NMSQT information has self-nomination or other local procedures. For been used by high school counselors to assist in many AP courses, students with moderate scores advising students in college planning, high school on the PSAT/NMSQT have a high probability of suc- course selection, and for scholarship awards. In- cess on the examinations. For example, a majority formation from the PSAT/NMSQT can also be very of students with PSAT/NMSQT verbal scores of useful for high schools in identifying additional 46–50 received grades of 3 or above on nearly all of students who may be successful in Advanced the 29 AP Examinations studied, while over one- Placement courses, and assisting schools in deter- third of students with scores of 41–45 achieved mining whether to offer additional Advanced grades of 3 or above on five AP Examinations. Placement courses. There are substantial variations across AP subjects that must be considered. -

Advanced Placement & Dual Enrollment Programs
MIDDLETOWN TOWNSHIP PUBLIC SCHOOLS ADVANCED PLACEMENT & DUAL ENROLLMENT PROGRAMS What are Advanced Placement courses? ● College-level curricula approved by the College Board to be offered at the high school level. ● Participating students experience the rigors of college-level studies with the support of the high school environment. ● Offer students the opportunity to earn college credit while in high school. “How do I know if a college gives college credit or course placement for AP scores?” - For Parents & Guardians - The College Board CLICK HERE Why should my child consider taking an AP course? Reasons Associated With Taking Advanced Placement Courses The Right Reasons Other Reasons 1. Students have a love of the 1. It looks good on a student’s subject. high school transcript. 2. Students want to learn more 2. Many of a student’s friends about the subject. may be taking the course. 3. Students have a strong desire 3. Students feel pressured to to go into more depth in this take the course. subject. 4. Students want to be challenged in the subject. What is Middletown’s philosophy regarding Advanced Placement? The Middletown Township School District believes that all students can benefit from the rigor of Advanced Placement coursework. We believe that access to our very best curricula should be open to all students willing to put forth the effort to succeed in college-level courses. By making our Advanced Placement program more inclusive, we believe that Middletown students will be part of a college-going culture that will help to ensure his or her readiness to succeed at the post-secondary level. -

Comprehensive Glossary
Comprehensive Glossary 2019–20 Texas Academic Performance Report Cover Page 2020 Accountability Rating: Given the impact of COVID-19, all districts and schools received a label of Not Rated: Declared State of Disaster for their 2020 accountability ratings. Click here to read the official announcement. 2020 Special Education Determination Status (district TAPR only): This label represents an integrated determination level status based on an evaluation of each local education agency’s (LEA) Results Driven Accountability (RDA) indicators in the special education program area and four Federally Required Elements (FREs), which include the State Performance Plan (SPP) compliance indicators 9, 10, 11, 12, and 13; data integrity; uncorrected noncompliance; and financial audit findings. Each LEA receives one of four special education determination levels (DLs): Meets Requirements Needs Assistance Needs Intervention Needs Substantial Intervention For additional information, please see the links below. General Information about RDA and SPP/APR: Results Driven Accountability (RDA): https://tea.texas.gov/academics/special-student- populations/review-and-support/results-driven-accountability-rda State Performance Plan and Annual Performance Report: https://tea.texas.gov/reports-and-data/data- submission/state-performance-plan#stateperformance Methodology for RDA and SPP/APR: 2020 RDA Manual: https://tea.texas.gov/student-assessment/monitoring-and-interventions/rda/rda- and-pbmas-manuals FFY 2018 SPP/APR Methodology: https://tea.texas.gov/sites/default/files/method%2018_19.pdf -

AP (Advanced Placement) As College Admissions Become
AP (Advanced Placement) As college admissions become increasingly competitive, any possible edge can be the difference between a coveted acceptance or a dreaded rejection. Advanced Placement (AP) is a program that offers college-level curriculum and examinations to high school students for them to prove their proficiency in certain subjects before starting college. Even though there is no specific rule of thumb to take any number of APs, statistics suggest that students applying for STEM courses on an average take 4-5 APs, which can also go up to 8 in number. Therefore, when you set a target for yourself, plan them well and spread them evenly throughout 10th, 11th and 12th standards, so that you don’t stress yourself by taking all of them in one single go. Please remember, APs are conducted by College Board for international students only once a year in May. Here is how you could plan your timeline ahead. ____________________________________________________________________________________________________ AP Calculus AB and AP Calculus BC What are AP Calculus AB and AP Calculus BC? ● Advanced Placement Calculus is a set of two distinct Advanced Placement Calculus courses and exams offered by College Board. Who should ideally take Calculus AB and AP Calculus BC? ● Calculus BC is recommended to students aspiring for STEM courses for undergrad. ● Calculus AB is recommended to students aspiring for Business, Economics courses for undergrad or the ones who are aspiring for STEM courses but have a time constraint. ● Calculus BC is a prereQuisite for applying for Stanford Pre-Collegiate University Level Online-Math and similar courses which students attend in 11th std. -

Spring 2019 Ap & Sat Subject Program Schedule Elite Prep Arcadia
SPRING 2019 AP & SAT SUBJECT PROGRAM SCHEDULE HYBRID AP & SAT SUBJECT AP EXAM PREP ONLY EXAM PREP CLASSES CLASSES March 1 - May 31, 2019 March 8 - May 11, 2019 These 14-week programs target both the AP exams in May These 10-week classes will focus ONLY on preparing and the SAT Subject exams in June. The first ten weeks of students for the 2019 AP examinations. Classes include a class will focus on preparing students for the AP exam and weekly practice test as well as lessons and test review. the last 4 weeks of class will focus on preparing for the SAT *Students must currently be enrolled in the corresponding Subject Exam taking place on June 1, 2019. AP classes at school to register for these programs. *Students must currently be enrolled in the corresponding AP classes at school to register for these programs. AP English Language & Composition AP Biology & Biology (M) SAT Subject Friday 4:00pm–7:00pm Friday 5:30pm–8:30pm AP Psychology Saturday 9:00am–12:00pm Friday 5:00pm–8:00pm AP Calculus AB & Math Level 2 SAT Subject AP Computer Science A Saturday 10:00am–1:00pm Please contact our office for more information. TBD AP Chemistry & Chemistry SAT Subject Friday 5:30pm–8:30pm Saturday 10:00am–1:00pm Saturday 1:00pm–4:00pm AP Physics 1 & Physics SAT Subject Saturday 10:00am–1:00pm SAT SUBJECT ONLY CLASSES AP US History & US History SAT Subject March 1 - May 31, 2019 Saturday 10:00am–1:00pm These 14-week classes will focus solely on the SAT Subject Exam and therefore are recommended for students who are *Students also have the option of taking ONLY the AP preparation very confident in the subject matter. -
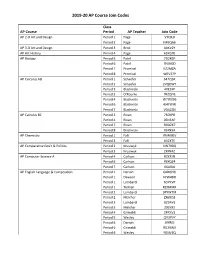
2019-20 AP Course Join Codes
2019-20 AP Course Join Codes Class AP Course Period AP Teacher Join Code AP 2-D Art and Design Period 1 Page VYQKJJ Period 2 Page N49QA6 AP 3-D Art and Design Period 3 Brod 4AKV2Y AP Art History Period 4 Page 63KG9E AP Biology Period 5 Patel 7G2K6P Period 6 Patel 7NJAQD Period 7 Promisel KZ2MZA Period 8 Promisel WEV37P AP Calculus AB Period 1 Schaefer 647Q6X Period 2 Schaefer 2VQDWY Period 3 Blachinski 4XEZ4P Period 3 O'Rourke 7RZQNE Period 4 Blachinski W7WX96 Period 6 Blachinski 4J4YWW Period 7 Blachinski VG42ZN AP Calculus BC Period 3 Rivan 7R7KPR Period 4 Rivan Z6N3AP Period 7 Rivan RGAZK7 Period 8 Blachinski VJ4N9A AP Chemistry Period 1 Falli YM4MEV Period 3 Falli 6X2X7E AP Comparative Gov't & Politics Period 2 Krusiwyk KW799Q Period 3 Krusiwyk JXXN4Z AP Computer Science A Period 4 Carlson RZXPJN Period 6 Carlson X6YQZ4 Period 7 Carlson V6ARAJ AP English Language & Composition Period 1 Darrah 6ARGNG Period 1 Dawson VYW4DD Period 1 Lombardi 6DYKVP Period 1 Tieman RZXMMR Period 2 Lombardi 9PYW7M Period 2 Melcher ZX6XQ3 Period 3 Lombardi GZ9AVE Period 3 Melcher 293Y93 Period 4 Grimaldi 2PPXV3 Period 5 Wesley QV3PVY Period 6 Darrah J9YREJ Period 6 Grimaldi RG29WX Period 6 Wesley YGNVEQ 2019-20 AP Course Join Codes Period 7 Darrah PW7GNN Period 7 Kulat A6KZAG Period 7 Tieman QA4WP6 Period 7 Wesley REW3XM Period 8 Tieman WEPWN4 AP English Literature & Composition Period 1 Lyons V3WJDJ Period 2 Lyons RVYMZ3 Period 4 Gutierrez PQPNQQ Period 6 Condon VJ4P6J Period 7 Condon Q4V6NE Period 7 Dudek WWGR2X Period 7 Gutierrez RRY76N Period 8 Dudek -

AP Chemistry
AP Chemistry Advanced Placement (AP) Chemistry is Introductory College Chemistry (ICC) • AP Chemistry is intended to allow students with advanced aptitude as well as academic / emotional maturity an opportunity to complete Introductory College Chemistry (ICC). • Rancho Bernardo’s AP Chemistry course is modeled upon the ICC curriculum at the Massachusetts Institute of Technology (MIT) and UC Berkley. • AP Chemistry is not a high school or college preparatory course. It is ICC and the course content covers the entire ICC curriculum at the same accelerated pace one would endure at any of the nations top ranked universities. 1 Who should consider AP Chemistry? • Students that intend to pursue a college curriculum that requires ICC should consider enrolling in AP Chemistry. • Depending upon the College or University one attends, the post-secondary curriculum majors and minors that typically require ICC are: • All branches of Chemistry and Biology; • Pre-Medical, Pre-Dental, Pre-Pharmacy & Pre-Vet Majors. • Various Engineering Degree programs. • Students that intend to apply to medical schools upon completion of their Baccalaureate studies are strongly encouraged to complete AP Chemistry prior to undergraduate matriculation. 2 Why should you consider AP Chemistry? • ICC is generally used as the initial “gatekeeper” course at most major universities in this country for careers in Science, Engineering, and Medicine. • Just passing ICC is not sufficient to qualify for admission in many of the most competitive post-baccalaureate programs. Applicants to these programs are generally expected to have maintained a level of achievement in the top quartile of their preparatory courses. • The objective of RB’s AP Chemistry course is to ensure that a student ranks in the upper percentile of the ICC course at the post-secondary institutions that they ultimately attend, including the most competitive in this country. -

A Guide to Standardized Testing-NEW-Final
CCO-MKA A Guide to Standardized Testing Standardized testing is far and away the most complicated and misunderstood part of the college admissions process. Some students even begin SAT testing at age 12 as part of summer talent identification programs at universities like Johns Hopkins. The testing doesn’t stop until winter of the senior year. But college admissions testing, most educators agree, is necessary. No one claims that a test of four hours is as important as classroom performance day in and day out, year in and year out. No two schools, however, are alike and the United States, lone among developed countries, has no national curriculum. It is impossible for colleges to compare the value of an A in rural Idaho with its counterpart in northern New Jersey. College admissions examinations have been developed as a national yardstick to help compare the performance of two students anywhere. This is an important concept to grasp. Because more and more students are presenting more and more honors grades to colleges, standardized test scores gain in importance as the only academic common denominator. Almost all MKA students sit for College Board examinations designed by the Educational Testing Service. These tests, briefly outlined below, include the PSAT, SAT Reasoning, SAT Subject, and Advanced Placement. In recent years, the ACT examination and its preliminary test, PLAN, have increased in popularity among MKA students. Test Academic Coverage Section Time Critical Reading: passages, sentence completion 2 50 min. PSAT Math: numbers, operations, algebra, functions, geometry, measurement, 2 50 min. data analysis, probability, statistics Writing Skills: sentence errors, sentence/paragraph improvement 1 30 min. -

ADVANCED PLACEMENT EXAMINATIONS Advanced Placement Credits Exempt a Student from Taking the Equivalent Courses at Hopkins
ADVANCED PLACEMENT EXAMINATIONS Advanced placement credits exempt a student from taking the equivalent courses at Hopkins. Johns Hopkins grants credit on some AP exams according to the accompanying table. If you receive an eligible score on any test listed on the accompanying table, please request that a score report for all years be sent to the Undergraduate Admissions Office at Hopkins. Remember that students are required to complete 100 credits through Johns Hopkins University. If a student enters the University with credit for an Advanced Placement examination and then completes an equivalent course at JHU for credit, the AP credits (and lab waiver, if applicable) will be disallowed. The credits and grade for the JHU course will appear on the academic record. The AP exam title remains on the record, but the credit value is reduced to zero. Biology: Students awarded credit for AP Biology are exempt from taking the corresponding lab courses. These lab courses are waived but no credit is awarded. Students awarded credit for AP Biology who complete 020.153 General Biology Lab I will lose 3 credits of AP Biology credit. Students awarded credit for AP Biology who complete 020.154 General Biology Lab II will lose 3 credits of AP Biology credit. Calculus: Students may receive credit for 110.108 Calculus I via only one test. Chemistry: Students awarded credit for AP Chemistry receive credit for the lecture and lab courses. Students awarded credit for AP Chemistry will lose a semester’s credit (4 credits) if either the corresponding lecture course or the corresponding lab course is completed at JHU. -

In What Year of High School Should Students Take AP Biology? Depending on the Program Sequence, Most Students Enroll in AP Biology During Their Junior Or Senior Year
In what year of high school should students take AP Biology? Depending on the program sequence, most students enroll in AP Biology during their junior or senior year. Since a first course in biology and a first course in chemistry are often prerequisites, this allows time for completion of those courses and also gives students time to gain math and laboratory skills. However, there are some schools that teach AP Biology at the sophomore level with no introductory biology course and highly motivated students have been very successful. The most important factor in student success seems to be maturity and motivation. Juniors often outperform seniors, especially as graduation time nears and "senioritis" sets in. -- Carolyn Schofield What course prerequisites should exist for students wishing to take AP Biology? Schools vary in their course prerequisites: Most consider Biology I to be a prerequisite, though some highly selective schools teach AP as the first and only course for their gifted sophomores. Completion of, or at least concurrent enrollment in, chemistry is an almost universal requirement. Many schools feel this ensures a certain level of maturity and helps students during difficult biochemistry sections. Some schools require that all three first-year science courses (biology, chemistry, and physics) be completed before a student may enroll in any AP science class. In doing so, their aim is to ensure basic competence before specialization. -- AP Program How much math does a student need before taking AP Biology? Math is a vital part of any science course, but trigonometry and calculus are definitely not mandatory. The following mathematical skills seem to be most helpful in the AP Biology course: algebra and geometry basics, rate and percent calculations, knowledge of scientific notation, basic probability that can be applied to statistical analysis, and knowledge of graphing (setup of axes and plotting of data). -

Princeton International School of Mathematics and Science
Princeton International School of Mathematics and Science Pengzhi Liu, Head of School Matthew Pearce, Executive Principal 19 Lambert Dr., Princeton, NJ 08540|(609) 454-5580|www.prismsus.org|CEEB code 311263 2017-2018 School Profile THE SCHOOL ADVANCED CURRICULUM Princeton International School of Math and Science All courses are taught at the honors, AP, and post-AP levels. (PRISMS), located in Princeton, NJ, is a comprehensive PRISMS students must take the following classes to meet four-year international boarding and day school graduation requirements: enrolling students in grades 9 to 12. PRISMS is accredited by Middle States. The school was founded in Subject Credits Subject Credits 2013, on the concept of combining the best of China’s STEM education with the best of America’s STEM Biology 1 Mathematics 4 education. PRISMS emphasizes the research and Chemistry 1 English 4 development process by reinforcing research skills, Physics 1 History 3 such as open-minded inquiry, problem-solving and STEM 3 Applied 2 innovation. Its first graduating class was in June 2016. Electives Engineering Fine Arts 1 Research & World 3 Development 2 Language ADMISSIONS PROCESS The PRISMS admissions process is selective. All 1 credit is equivalent to 1 academic year length (2 semesters). applicants are interviewed and screened thoroughly. A completed file consists of an application, transcripts, Advanced Placement Course Offerings: teacher recommendations, standardized testing - AP Biology SSAT (Secondary School Admissions Test) and • interview. In addition, all international students must • AP Calculus BC submit the TOEFL (Test of English as a Foreign • AP Chemistry Language) with required scores of 100 or higher. -
Pre-AP® Chemistry Course Guide
INCLUDES Approach to ® teaching and learning Pre-AP Course map Course framework Sample Chemistry assessment questions COURSE GUIDE preap.org/Chemistry-CG © 2021 College Board. 01560-064 01560-064-Pre-AP-Covers-3P.indd 79-80 3/27/20 1:38 PM Pre-AP® Chemistry COURSE GUIDE Please visit Pre-AP online at preap.collegeboard.org for more information and updates about the course and program features. ABOUT COLLEGE BOARD College Board is a mission-driven not-for-profit organization that connects students to college success and opportunity. Founded in 1900, College Board was created to expand access to higher education. Today, the membership association is made up of over 6,000 of the world’s leading educational institutions and is dedicated to promoting excellence and equity in education. Each year, College Board helps more than seven million students prepare for a successful transition to college through programs and services in college readiness and college success—including the SAT® and the Advanced Placement Program®. The organization also serves the education community through research and advocacy on behalf of students, educators, and schools. For further information, visit www.collegeboard.org. PRE-AP EQUITY AND ACCESS POLICY College Board believes that all students deserve engaging, relevant, and challenging grade- level coursework. Access to this type of coursework increases opportunities for all students, including groups that have been traditionally underrepresented in AP and college classrooms. Therefore, the Pre-AP program is dedicated to collaborating with educators across the country to ensure all students have the supports to succeed in appropriately challenging classroom experiences that allow students to learn and grow.