KLF2 Repressed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

KLF2 Induced
UvA-DARE (Digital Academic Repository) The transcription factor KLF2 in vascular biology Boon, R.A. Publication date 2008 Link to publication Citation for published version (APA): Boon, R. A. (2008). The transcription factor KLF2 in vascular biology. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:23 Sep 2021 Supplementary data: Genes induced by KLF2 Dekker et al. LocusLink Accession Gene Sequence Description Fold p-value ID number symbol change (FDR) 6654 AK022099 SOS1 cDNA FLJ12037 fis, clone HEMBB1001921. 100.00 5.9E-09 56999 AF086069 ADAMTS9 full length insert cDNA clone YZ35C05. 100.00 1.2E-09 6672 AF085934 SP100 full length insert cDNA clone YR57D07. 100.00 6.7E-13 9031 AF132602 BAZ1B Williams Syndrome critical region WS25 mRNA, partial sequence. -

Environmental Influences on Endothelial Gene Expression
ENDOTHELIAL CELL GENE EXPRESSION John Matthew Jeff Herbert Supervisors: Prof. Roy Bicknell and Dr. Victoria Heath PhD thesis University of Birmingham August 2012 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Tumour angiogenesis is a vital process in the pathology of tumour development and metastasis. Targeting markers of tumour endothelium provide a means of targeted destruction of a tumours oxygen and nutrient supply via destruction of tumour vasculature, which in turn ultimately leads to beneficial consequences to patients. Although current anti -angiogenic and vascular targeting strategies help patients, more potently in combination with chemo therapy, there is still a need for more tumour endothelial marker discoveries as current treatments have cardiovascular and other side effects. For the first time, the analyses of in-vivo biotinylation of an embryonic system is performed to obtain putative vascular targets. Also for the first time, deep sequencing is applied to freshly isolated tumour and normal endothelial cells from lung, colon and bladder tissues for the identification of pan-vascular-targets. Integration of the proteomic, deep sequencing, public cDNA libraries and microarrays, delivers 5,892 putative vascular targets to the science community. -

Angiogenic Patterning by STEEL, an Endothelial-Enriched Long
Angiogenic patterning by STEEL, an endothelial- enriched long noncoding RNA H. S. Jeffrey Mana,b, Aravin N. Sukumara,b, Gabrielle C. Lamc,d, Paul J. Turgeonb,e, Matthew S. Yanb,f, Kyung Ha Kub,e, Michelle K. Dubinskya,b, J. J. David Hob,f, Jenny Jing Wangb,e, Sunit Dasg,h, Nora Mitchelli, Peter Oettgeni, Michael V. Seftonc,d,j, and Philip A. Marsdena,b,e,f,1 aInstitute of Medical Science, University of Toronto, Toronto, ON M5S 1A8, Canada; bKeenan Research Centre for Biomedical Science in the Li Ka Shing Knowledge Institute, St. Michael’s Hospital, University of Toronto, Toronto, ON M5B 1T8, Canada; cDonnelly Centre for Cellular and Biomolecular Research, University of Toronto, Toronto, ON M5S 3E2, Canada; dInstitute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, ON M5S 3G9, Canada; eDepartment of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, ON M5S 1A8, Canada; fDepartment of Medical Biophysics, University of Toronto, Toronto, ON M5G 1L7, Canada; gArthur and Sonia Labatt Brain Tumour Research Institute, Hospital for SickKids, University of Toronto, Toronto, ON M5G 1X8, Canada; hDivision of Neurosurgery and Keenan Research Centre for Biomedical Science, St. Michael’s Hospital, University of Toronto, Toronto, ON M5B 1W8, Canada; iDepartment of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02115; and jDepartment of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, ON M5S 3E5, Canada Edited by Napoleone Ferrara, University of California, San Diego, La Jolla, CA, and approved January 24, 2018 (received for review August 28, 2017) Endothelial cell (EC)-enriched protein coding genes, such as endothelial formation in vitro and blood vessel formation in vivo. -

Multi-Targeted Mechanisms Underlying the Endothelial Protective Effects of the Diabetic-Safe Sweetener Erythritol
Multi-Targeted Mechanisms Underlying the Endothelial Protective Effects of the Diabetic-Safe Sweetener Erythritol Danie¨lle M. P. H. J. Boesten1*., Alvin Berger2.¤, Peter de Cock3, Hua Dong4, Bruce D. Hammock4, Gertjan J. M. den Hartog1, Aalt Bast1 1 Department of Toxicology, Maastricht University, Maastricht, The Netherlands, 2 Global Food Research, Cargill, Wayzata, Minnesota, United States of America, 3 Cargill RandD Center Europe, Vilvoorde, Belgium, 4 Department of Entomology and UCD Comprehensive Cancer Center, University of California Davis, Davis, California, United States of America Abstract Diabetes is characterized by hyperglycemia and development of vascular pathology. Endothelial cell dysfunction is a starting point for pathogenesis of vascular complications in diabetes. We previously showed the polyol erythritol to be a hydroxyl radical scavenger preventing endothelial cell dysfunction onset in diabetic rats. To unravel mechanisms, other than scavenging of radicals, by which erythritol mediates this protective effect, we evaluated effects of erythritol in endothelial cells exposed to normal (7 mM) and high glucose (30 mM) or diabetic stressors (e.g. SIN-1) using targeted and transcriptomic approaches. This study demonstrates that erythritol (i.e. under non-diabetic conditions) has minimal effects on endothelial cells. However, under hyperglycemic conditions erythritol protected endothelial cells against cell death induced by diabetic stressors (i.e. high glucose and peroxynitrite). Also a number of harmful effects caused by high glucose, e.g. increased nitric oxide release, are reversed. Additionally, total transcriptome analysis indicated that biological processes which are differentially regulated due to high glucose are corrected by erythritol. We conclude that erythritol protects endothelial cells during high glucose conditions via effects on multiple targets. -

1 Supporting Information for a Microrna Network Regulates
Supporting Information for A microRNA Network Regulates Expression and Biosynthesis of CFTR and CFTR-ΔF508 Shyam Ramachandrana,b, Philip H. Karpc, Peng Jiangc, Lynda S. Ostedgaardc, Amy E. Walza, John T. Fishere, Shaf Keshavjeeh, Kim A. Lennoxi, Ashley M. Jacobii, Scott D. Rosei, Mark A. Behlkei, Michael J. Welshb,c,d,g, Yi Xingb,c,f, Paul B. McCray Jr.a,b,c Author Affiliations: Department of Pediatricsa, Interdisciplinary Program in Geneticsb, Departments of Internal Medicinec, Molecular Physiology and Biophysicsd, Anatomy and Cell Biologye, Biomedical Engineeringf, Howard Hughes Medical Instituteg, Carver College of Medicine, University of Iowa, Iowa City, IA-52242 Division of Thoracic Surgeryh, Toronto General Hospital, University Health Network, University of Toronto, Toronto, Canada-M5G 2C4 Integrated DNA Technologiesi, Coralville, IA-52241 To whom correspondence should be addressed: Email: [email protected] (M.J.W.); yi- [email protected] (Y.X.); Email: [email protected] (P.B.M.) This PDF file includes: Materials and Methods References Fig. S1. miR-138 regulates SIN3A in a dose-dependent and site-specific manner. Fig. S2. miR-138 regulates endogenous SIN3A protein expression. Fig. S3. miR-138 regulates endogenous CFTR protein expression in Calu-3 cells. Fig. S4. miR-138 regulates endogenous CFTR protein expression in primary human airway epithelia. Fig. S5. miR-138 regulates CFTR expression in HeLa cells. Fig. S6. miR-138 regulates CFTR expression in HEK293T cells. Fig. S7. HeLa cells exhibit CFTR channel activity. Fig. S8. miR-138 improves CFTR processing. Fig. S9. miR-138 improves CFTR-ΔF508 processing. Fig. S10. SIN3A inhibition yields partial rescue of Cl- transport in CF epithelia. -

Inhibition of Adipocyte Differentiation by Rorα
FEBS Letters 583 (2009) 2031–2036 journal homepage: www.FEBSLetters.org Inhibition of adipocyte differentiation by RORa Hélène Duez a,b,c,1, Christian Duhem a,b,c,1, Saara Laitinen a,b,c, Prashant S. Patole a,b,c, Mouaadh Abdelkarim a,b,c, Brigitte Bois-Joyeux d, Jean-Louis Danan d, Bart Staels a,b,c,* a Institut Pasteur de Lille, Département d’Athérosclérose, Lille F-59019, France b INSERM UMR 545, Lille F-59019, France c Université Lille Nord de France, Faculté des Sciences Pharmaceutiques et Biologiques et Faculté de Médecine, Lille F-59006, France d Centre National de la Recherche Scientifique FRE3210, Faculté de Médecine René Descartes Paris 5, 75015 Paris, France article info abstract Article history: Here we show that gene expression of the nuclear receptor RORa is induced during adipogenesis, Received 8 April 2009 with RORa4 being the most abundantly expressed isoform in human and murine adipose tissue. Revised 27 April 2009 Over-expression of RORa4 in 3T3-L1 cells impairs adipogenesis as shown by the decreased expres- Accepted 8 May 2009 sion of adipogenic markers and lipid accumulation, accompanied by decreased free fatty acid and Available online 18 May 2009 glucose uptake. By contrast, mouse embryonic fibroblasts from staggerer mice, which carry a muta- Edited by Robert Barouki tion in the RORa gene, differentiate more efficiently into mature adipocytes compared to wild-type cells, a phenotype which is reversed by ectopic RORa4 restoration. Ó 2009 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved. Keywords: Adipogenesis RORa Nuclear receptors RAR-related orphan receptors (RORs) 1. -

Regulation of T Follicular Helper Cells by ICOS
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 6, No. 26 Editorial Regulation of T follicular helper cells by ICOS Andreas Hutloff T follicular helper (TFH) cells are gatekeepers of the (OPN-i), which facilitates nuclear translocation [3]. In the humoral immune response. Without help from this CD4+ nucleus, OPN-i dimerizes with Bcl-6 and protects it from T cell subset, B cells cannot differentiate into high-affinity proteasomal degradation. However, this OPN-i and the memory B cells and antibody-producing long-lived plasma above Klf2 pathway do act at different times. Upon ICOS cells which are the basis of protective immune responses. signaling blockade, Klf2 is upregulated within a few hours At the same time, dysregulated TFH cell responses are and TFH cells lose their typical homing receptor pattern in causative for many autoimmune disorders (reviewed in less than 24 hours [6]. In contrast, complete degradation [1]). The inducible T cell costimulator ICOS, which is of Bcl-6 takes several days [3, 6]. Therefore, the ICOS - structurally and functionally related to CD28, has been Klf2 axis first leads to emigration of TFH cells out of the known for many years as an important regulator of TFH B cell follicle (and thereby to a loss of function), whereas cells. ICOS knock-out mice as well as ICOS-deficient the osteopontin pathway acts as a second strike pathway patients have only few TFH cells and very small germinal eliminating the lineage-defining transcription factor Bcl-6. centers upon immunization, which results in severely Another important finding especially for potential compromised antigen-specific immunoglobulin levels therapeutic applications is that ICOS and the structurally and the phenotype of common variable immunodeficiency related CD28 molecule act in different phases of TFH cell in humans (reviewed in [2]). -

Beta Cell Adaptation to Pregnancy Requires Prolactin Action on Both
www.nature.com/scientificreports OPEN Beta cell adaptation to pregnancy requires prolactin action on both beta and non‑beta cells Vipul Shrivastava1, Megan Lee1, Daniel Lee1, Marle Pretorius1, Bethany Radford1, Guneet Makkar1 & Carol Huang1,2,3* Pancreatic islets adapt to insulin resistance of pregnancy by up regulating β‑cell mass and increasing insulin secretion. Previously, using a transgenic mouse with global, heterozygous deletion of prolactin receptor (Prlr+/−), we found Prlr signaling is important for this adaptation. However, since Prlr is expressed in tissues outside of islets as well as within islets and prolactin signaling afects β‑cell development, to understand β‑cell‑specifc efect of prolactin signaling in pregnancy, we generated a transgenic mouse with an inducible conditional deletion of Prlr from β‑cells. Here, we found that β‑cell‑specifc Prlr reduction in adult mice led to elevated blood glucose, lowed β‑cell mass and blunted in vivo glucose‑stimulated insulin secretion during pregnancy. When we compared gene expression profle of islets from transgenic mice with global (Prlr+/−) versus β‑cell‑specifc Prlr reduction (βPrlR+/−), we found 95 diferentially expressed gene, most of them down regulated in the Prlr+/− mice in comparison to the βPrlR+/− mice, and many of these genes regulate apoptosis, synaptic vesicle function and neuronal development. Importantly, we found that islets from pregnant Prlr+/− mice are more vulnerable to glucolipotoxicity‑induced apoptosis than islets from pregnant βPrlR+/− mice. These observations suggest that down regulation of prolactin action during pregnancy in non‑β‑cells secondarily and negatively afect β‑cell gene expression, and increased β‑cell susceptibility to external insults. -
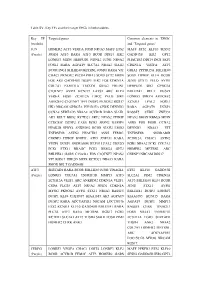
Targeted Genes Common Elements In
Table SV. Key TFs and their target DEGs in hub modules. Key TF Targeted genes Common elements in ‘DEGs’ (module) and ‘Targeted genes’ JUN HOMER2 ATF3 VEGFA FOSB NR4A3 MAFF ETS2 MAFF ETS2 KLF10 SESN2 (Purple) JOSD1 ATF3 RARA ATF3 BCOR DDIT4 IER2 GADD45B IER2 GPT2 LONRF3 MIDN HERPUD1 NDNL2 JUNB NR4A2 PHACTR3 DDIT4 ING1 SKP2 FOSL1 RARA AGPAT9 SLC7A1 NR4A2 SIAH2 CDKN1A VEGFA ATF3 BCOR ING1 BHLHE40 METRNL JOSD1 RARA VIT GRIA2 PPP1R15A BHLHE40 CHAC1 PKNOX2 PLCB4 PHF13 SOX9 SYT2 MIDN SOX9 ITPRIP KLF4 BCOR FOS AK5 GADD45B DUSP1 IER2 FOS CDKN1A JUNB STX11 PELO AVPI1 COL7A1 FAM131A TBCCD1 GRIA2 ERLIN1 HERPUD1 SIK1 GPRC5A C1QTNF7 AVPI1 KCNJ15 LATS2 ARC KLF4 BHLHE41 RELT DUSP2 VEGFA FOSB ZC3H12A LMO2 PELO SIK1 LONRF3 SPRY4 ARHGEF2 ARHGEF2 C1QTNF7 TFPI DUSP2 PKNOX2 RGS17 KCNJ15 LPAL2 FOSL1 HK1 NRCAM GPRC5A PPP1R15A CERK DENND3 RARA AGPAT9 DUSP1 CCNA2 SERTAD3 NR4A2 ACVR1B RARA SIAH1 RASSF5 CERK ZNF331 AK5 RELT BRD2 KCTD21 SKP2 NPAS2 ITPRIP NPAS2 SMOX RBM24 MIDN CCDC85C DUSP2 CARS EGR1 SESN2 RASSF5 ASNS FOS FOSB CCNA2 PIP4K2B SPRY4 ANKRD52 BCOR SIAH2 LMO2 DENND3 NR4A3 VIT TNFRSF1B ASTN2 PHACTR3 ASNS FEM1C TNFRSF1B SNORA80B CSRNP3 ITPRIP BNIP3L ATF3 ZNF331 RARA ZC3H12A CHAC1 ASTN2 VEZF1 DUSP1 SNORA80B KLF10 LPAL2 UBE2O EGR1 NR4A2 PCK1 COL7A1 PCK1 STX11 RRAGC PCK1 RBM24 GPT2 HOMER2 METRNL ARC BHLHE41 GARS C15orf41 FOS C1QTNF7 NPAS2 CSRNP3 NRCAM RGS17 VIT RGS17 UBE2O SOX9 KCTD21 NR4A3 RARA SMOX RELT GADD45B ATF3 SERTAD1 RARA BCOR BHLHE40 JUNB TINAGL1 ETS2 KLF10 GADD45B (Purple) LONRF3 COL7A1 TNFRSF1B MMP13 ATF3 SLC2A1 PIM2 CDKN1A ZC3H12A VEZF1 ARC ANKRD52 -

Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis
International Journal of Molecular Sciences Review Circadian Rhythm: Potential Therapeutic Target for Atherosclerosis and Thrombosis Andy W. C. Man , Huige Li and Ning Xia * Department of Pharmacology, Johannes Gutenberg University Medical Center, 55131 Mainz, Germany; [email protected] (A.W.C.M.); [email protected] (H.L.) * Correspondence: [email protected] Abstract: Every organism has an intrinsic biological rhythm that orchestrates biological processes in adjusting to daily environmental changes. Circadian rhythms are maintained by networks of molecular clocks throughout the core and peripheral tissues, including immune cells, blood vessels, and perivascular adipose tissues. Recent findings have suggested strong correlations between the circadian clock and cardiovascular diseases. Desynchronization between the circadian rhythm and body metabolism contributes to the development of cardiovascular diseases including arterioscle- rosis and thrombosis. Circadian rhythms are involved in controlling inflammatory processes and metabolisms, which can influence the pathology of arteriosclerosis and thrombosis. Circadian clock genes are critical in maintaining the robust relationship between diurnal variation and the cardio- vascular system. The circadian machinery in the vascular system may be a novel therapeutic target for the prevention and treatment of cardiovascular diseases. The research on circadian rhythms in cardiovascular diseases is still progressing. In this review, we briefly summarize recent studies on circadian rhythms and cardiovascular homeostasis, focusing on the circadian control of inflammatory processes and metabolisms. Based on the recent findings, we discuss the potential target molecules for future therapeutic strategies against cardiovascular diseases by targeting the circadian clock. Keywords: clock genes; inflammation; oxidative stress; circadian disruption; cardiovascular diseases Citation: Man, A.W.C.; Li, H.; Xia, N. -

WO 2016/040794 Al 17 March 2016 (17.03.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/040794 Al 17 March 2016 (17.03.2016) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, C12N 1/19 (2006.01) C12Q 1/02 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, C12N 15/81 (2006.01) C07K 14/47 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US20 15/049674 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 11 September 2015 ( 11.09.201 5) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/050,045 12 September 2014 (12.09.2014) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: WHITEHEAD INSTITUTE FOR BIOMED¬ DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, ICAL RESEARCH [US/US]; Nine Cambridge Center, LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, Cambridge, Massachusetts 02142-1479 (US). -

Factor Expression and Correlate with Specific Transcription in Early Human Precursor B Cell Subsets Ig Gene Rearrangement Steps
The Journal of Immunology Ig Gene Rearrangement Steps Are Initiated in Early Human Precursor B Cell Subsets and Correlate with Specific Transcription Factor Expression1 Menno C. van Zelm,*† Mirjam van der Burg,* Dick de Ridder,*‡ Barbara H. Barendregt,*† Edwin F. E. de Haas,* Marcel J. T. Reinders,‡ Arjan C. Lankester,§ Tom Re´ve´sz,¶ Frank J. T. Staal,* and Jacques J. M. van Dongen2* The role of specific transcription factors in the initiation and regulation of Ig gene rearrangements has been studied extensively in mouse models, but data on normal human precursor B cell differentiation are limited. We purified five human precursor B cell subsets, and assessed and quantified their IGH, IGK, and IGL gene rearrangement patterns and gene expression profiles. Pro-B cells already massively initiate DH-JH rearrangements, which are completed with VH-DJH rearrangements in pre-B-I cells. Large cycling pre-B-II cells are selected for in-frame IGH gene rearrangements. The first IGK/IGL gene rearrangements were initiated in pre-B-I cells, but their frequency increased enormously in small pre-B-II cells, and in-frame selection was found in immature B cells. Transcripts of the RAG1 and RAG2 genes and earlier defined transcription factors, such as E2A, early B cell factor, E2-2, PAX5, and IRF4, were specifically up-regulated at stages undergoing Ig gene rearrangements. Based on the combined Ig gene rearrangement status and gene expression profiles of consecutive precursor B cell subsets, we identified 16 candidate genes involved in initiation and/or regulation of Ig gene rearrangements. These analyses provide new insights into early human pre- cursor B cell differentiation steps and represent an excellent template for studies on oncogenic transformation in precursor B acute lymphoblastic leukemia and B cell differentiation blocks in primary Ab deficiencies.