The Risk of Dengue for Non-Immune Foreign Visitors to the 2016 Summer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Disability Classification System
CLASSIFICATION SYSTEM FOR STUDENTS WITH A DISABILITY Track & Field (NB: also used for Cross Country where applicable) Current Previous Definition Classification Classification Deaf (Track & Field Events) T/F 01 HI 55db loss on the average at 500, 1000 and 2000Hz in the better Equivalent to Au2 ear Visually Impaired T/F 11 B1 From no light perception at all in either eye, up to and including the ability to perceive light; inability to recognise objects or contours in any direction and at any distance. T/F 12 B2 Ability to recognise objects up to a distance of 2 metres ie below 2/60 and/or visual field of less than five (5) degrees. T/F13 B3 Can recognise contours between 2 and 6 metres away ie 2/60- 6/60 and visual field of more than five (5) degrees and less than twenty (20) degrees. Intellectually Disabled T/F 20 ID Intellectually disabled. The athlete’s intellectual functioning is 75 or below. Limitations in two or more of the following adaptive skill areas; communication, self-care; home living, social skills, community use, self direction, health and safety, functional academics, leisure and work. They must have acquired their condition before age 18. Cerebral Palsy C2 Upper Severe to moderate quadriplegia. Upper extremity events are Wheelchair performed by pushing the wheelchair with one or two arms and the wheelchair propulsion is restricted due to poor control. Upper extremity athletes have limited control of movements, but are able to produce some semblance of throwing motion. T/F 33 C3 Wheelchair Moderate quadriplegia. Fair functional strength and moderate problems in upper extremities and torso. -

IPC Accessibility Guide
2 TABLE OF CONTENTS FIGURES AND TABLES ................................................................................................................. 8 Foreword ........................................................................................................................................... 10 Introduction ................................................................................................................................. 10 Evolving content ......................................................................................................................... 10 Disclosure ...................................................................................................................................... 11 Structure and content of the IPC Accessibility Guide ...................................................... 11 Content ........................................................................................................................................... 11 Executive summary ......................................................................................................................... 12 Aim and purpose of the Guide ................................................................................................ 12 Key objectives of the Guide ..................................................................................................... 12 Target audience of the Guide ................................................................................................. 12 1 General information -

Ifds Functional Classification System & Procedures
IFDS FUNCTIONAL CLASSIFICATION SYSTEM & PROCEDURES MANUAL 2009 - 2012 Effective – 1 January 2009 Originally Published – March 2009 IFDS, C/o ISAF UK Ltd, Ariadne House, Town Quay, Southampton, Hampshire, SO14 2AQ, GREAT BRITAIN Tel. +44 2380 635111 Fax. +44 2380 635789 Email: [email protected] Web: www.sailing.org/disabled 1 Contents Page Introduction 5 Part A – Functional Classification System Rules for Sailors A1 General Overview and Sailor Evaluation 6 A1.1 Purpose 6 A1.2 Sailing Functions 6 A1.3 Ranking of Functional Limitations 6 A1.4 Eligibility for Competition 6 A1.5 Minimum Disability 7 A2 IFDS Class and Status 8 A2.1 Class 8 A2.2 Class Status 8 A2.3 Master List 10 A3 Classification Procedure 10 A3.0 Classification Administration Fee 10 A3.1 Personal Assistive Devices 10 A3.2 Medical Documentation 11 A3.3 Sailors’ Responsibility for Classification Evaluation 11 A3.4 Sailor Presentation for Classification Evaluation 12 A3.5 Method of Assessment 12 A3.6 Deciding the Class 14 A4 Failure to attend/Non Co-operation/Misrepresentation 16 A4.1 Sailor Failure to Attend Evaluation 16 A4.2 Non Co-operation during Evaluation 16 A4.3 International Misrepresentation of Skills and/or Abilities 17 A4.4 Consequences for Sailor Support Personnel 18 A4.5 Consequences for Teams 18 A5 Specific Rules for Boat Classes 18 A5.1 Paralympic Boat Classes 18 A5.2 Non-Paralympic Boat Classes 19 Part B – Protest and Appeals B1 Protest 20 B1.1 General Principles 20 B1.2 Class Status and Protest Opportunities 21 B1.3 Parties who may submit a Classification Protest -
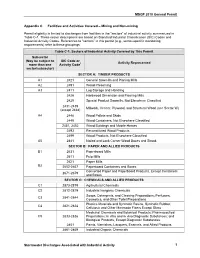
Facility SIC Code Table
MSGP 2010 General Permit Appendix C. Facilities and Activities Covered – Mining and Non-mining Permit eligibility is limited to discharges from facilities in the “sectors” of industrial activity summarized in Table C-1. These sector descriptions are based on Standard Industrial Classification (SIC) Codes and Industrial Activity Codes. References to “sectors” in this permit (e.g., sector-specific monitoring requirements) refer to these groupings. Table C-1. Sectors of Industrial Activity Covered by This Permit Subsector (May be subject to SIC Code or Activity Represented more than one Activity Code1 sector/subsector) SECTOR A: TIMBER PRODUCTS A1 2421 General Sawmills and Planing Mills A2 2491 Wood Preserving A3 2411 Log Storage and Handling 2426 Hardwood Dimension and Flooring Mills 2429 Special Product Sawmills, Not Elsewhere Classified 2431-2439 Millwork, Veneer, Plywood, and Structural Wood (see Sector W) (except 2434) A4 2448 Wood Pallets and Skids 2449 Wood Containers, Not Elsewhere Classified 2451, 2452 Wood Buildings and Mobile Homes 2493 Reconstituted Wood Products 2499 Wood Products, Not Elsewhere Classified A5 2441 Nailed and Lock Corner Wood Boxes and Shook SECTOR B: PAPER AND ALLIED PRODUCTS B1 2631 Paperboard Mills 2611 Pulp Mills 2621 Paper Mills B2 2652-2657 Paperboard Containers and Boxes Converted Paper and Paperboard Products, Except Containers 2671-2679 and Boxes SECTOR C: CHEMICALS AND ALLIED PRODUCTS C1 2873-2879 Agricultural Chemicals C2 2812-2819 Industrial Inorganic Chemicals Soaps, Detergents, and Cleaning Preparations; -

United States Olympic Committee and U.S. Department of Veterans Affairs
SELECTION STANDARDS United States Olympic Committee and U.S. Department of Veterans Affairs Veteran Monthly Assistance Allowance Program The U.S. Olympic Committee supports Paralympic-eligible military veterans in their efforts to represent the USA at the Paralympic Games and other international sport competitions. Veterans who demonstrate exceptional sport skills and the commitment necessary to pursue elite-level competition are given guidance on securing the training, support, and coaching needed to qualify for Team USA and achieve their Paralympic dreams. Through a partnership between the United States Department of Veterans Affairs and the USOC, the VA National Veterans Sports Programs & Special Events Office provides a monthly assistance allowance for disabled Veterans of the Armed Forces training in a Paralympic sport, as authorized by 38 U.S.C. § 322(d) and section 703 of the Veterans’ Benefits Improvement Act of 2008. Through the program the VA will pay a monthly allowance to a Veteran with a service-connected or non-service-connected disability if the Veteran meets the minimum VA Monthly Assistance Allowance (VMAA) Standard in his/her respective sport and sport class at a recognized competition. Athletes must have established training and competition plans and are responsible for turning in monthly and/or quarterly forms and reports in order to continue receiving the monthly assistance allowance. Additionally, an athlete must be U.S. citizen OR permanent resident to be eligible. Lastly, in order to be eligible for the VMAA athletes must undergo either national or international classification evaluation (and be found Paralympic sport eligible) within six months of being placed on the allowance pay list. -

Para Cycling Information Sheet About the Sport Classification Explained
Para cycling information sheet About the sport Para cycling is cycling for people with impairments resulting from a health condition (disability). Para athletes with physical impairments either compete on handcycles, tricycles or bicycles, while those with a visual impairment compete on tandems with a sighted ‘pilot’. Para cycling is divided into track and road events, with seven events in total. Classification explained In Para sport classification provides the structure for fair and equitable competition to ensure that winning is determined by skill, fitness, power, endurance, tactical ability and mental focus – the same factors that account for success in sport for able-bodied athletes. The Para sport classification assessment process identifies the eligibility of each Para athlete’s impairment, and groups them into a sport class according to the degree of activity limitation resulting from their impairment. Classification is sport-specific as an eligible impairment affects a Para athlete’s ability to perform in different sports to a different extent. Each Para sport has a different classification system. Standard Classification in detail Para-Cycling sport classes include: Handcycle sport classes H1 – 5: There are five different sport classes for handcycle racing. The lower numbers indicate a more severe activity limitation. Para athletes competing in the H1 classes have a complete loss of trunk and leg function and limited arm function, e.g. as a result of a spinal cord injury. Para athletes in the H4 class have limited or no leg function, but good trunk and arm function. Para cyclists in sport classes H1 – 4 compete in a reclined position. Para cyclists in the H5 sport class sit on their knees because they are able to use their arms and trunk to accelerate the handcycle. -

(VA) Veteran Monthly Assistance Allowance for Disabled Veterans
Revised May 23, 2019 U.S. Department of Veterans Affairs (VA) Veteran Monthly Assistance Allowance for Disabled Veterans Training in Paralympic and Olympic Sports Program (VMAA) In partnership with the United States Olympic Committee and other Olympic and Paralympic entities within the United States, VA supports eligible service and non-service-connected military Veterans in their efforts to represent the USA at the Paralympic Games, Olympic Games and other international sport competitions. The VA Office of National Veterans Sports Programs & Special Events provides a monthly assistance allowance for disabled Veterans training in Paralympic sports, as well as certain disabled Veterans selected for or competing with the national Olympic Team, as authorized by 38 U.S.C. 322(d) and Section 703 of the Veterans’ Benefits Improvement Act of 2008. Through the program, VA will pay a monthly allowance to a Veteran with either a service-connected or non-service-connected disability if the Veteran meets the minimum military standards or higher (i.e. Emerging Athlete or National Team) in his or her respective Paralympic sport at a recognized competition. In addition to making the VMAA standard, an athlete must also be nationally or internationally classified by his or her respective Paralympic sport federation as eligible for Paralympic competition. VA will also pay a monthly allowance to a Veteran with a service-connected disability rated 30 percent or greater by VA who is selected for a national Olympic Team for any month in which the Veteran is competing in any event sanctioned by the National Governing Bodies of the Olympic Sport in the United State, in accordance with P.L. -

VISTA2013 Scientific Conference Booklet Gustav-Stresemann-Institut Bonn, 1-4 May 2013
International Paralympic Committee VISTA2013 Scientific Conference Booklet Gustav-Stresemann-Institut Bonn, 1-4 May 2013 “Equipment & Technology in Paralympic Sports” “Equipment & Technology in Paralympic Sports” VISTA2013 Scientific Conference Gustav-Stresemann-Institut Bonn, 1-4 May 2013 The VISTA2013 Conference is organised by: International Paralympic Committee Adenauerallee 212-214 53113 Bonn, Germany Tel. +49 228 2097-200 Fax +49 228 2097-209 [email protected] www.paralympic.org © 2013 International Paralympic Committee I 2 I VISTA2013 Scientific Conference Table of Contents Forewords 4 VISTA2013 Scientific Committee 6 General Information 7 Venue 8 Programme at a Glance 10 Scientific Programme – Detail 12 Keynote Speakers 21 Symposia - Abstracts 26 Free Communications - Abstracts 32 Free Communications - Posters 78 Scientific Information 102 Scientific Award Winner 103 I 3 I VISTA2013 Scientific Conference Forewords Sir Philip Craven, MBE President, International Paralympic Committee Dear participants, On behalf of the International Paralympic Committee (IPC), I would like to welcome you to the 2013 VISTA Conference, the IPC’s scientific conference that will this year centre around the equipment and technology used in Paralympic sport. This conference brings together some of the world’s leading sport scientists, administrators, coaches and athletes. We hope you can take what you learn over the next few days back home with you to your respective communities to help further advance the Paralympic Movement. The next few days will include keynote addresses, symposia, oral presentations and poster sessions put together by the IPC Sports Science Committee that will motivate and influence you in your respective work environments, no matter which part of the Paralympic Movement you represent. -

Should the Para-Cycling Classification System Be Reclassified?
Should the para-cycling classification system be reclassified? David Borg1, John Osborne2, Johanna Liljedahl3, Michele Foster1, Carla Nooijen3 1The Hopkins Centre, Menzies Health Institute Queensland, Griffith University, Brisbane, Australia. 2School of Exercise and Nutrition Sciences, Queensland University of Technology, Brisbane, Australia. 3The Swedish School of Sport and Health Sciences (GIH), Stockholm, Sweden. Introduction Results Para-cycling athletes compete on bicycles (C1–5), handcycles The number of men in C1, C2, C3, C4 and C5 was 32, 76, 63, (H1–5) and tricycles (T1-2) in classes based on their functional 76, and 87, respectively. Nineteen men competed in T1 and 58 disability. Higher classes reflect less functional impairment. Para- in T2. The age range of men was 14–62 years. cycling classification is governed by the Union Cycliste Inter- The number of women competing in C1, C2, C3, C4 and C5 was nationale (UCI). The system aims to promote participation in the 4, 18, 16, 20 and 28, respectively. Eleven competed in T1 and sport, by controlling for the impact of impairment on the outcome 15 in T2. Women’s age ranged 17–55 years. of competition. Road race velocity for the bicycling and tricycling is shown in Recently, the separation between adjacent handcycling classes for Figure 1. Comparisons between adjacent classes for men and official UCI events was described––providing benchmarks for women are displayed in Table 2. active and potential athletes. Unfortunately, similar evaluation of the bicycling and tricycling disciplines has not been considered. With the expectation of C4 and C5 for women, the analysis showed that men's and women's road race performance was This study aimed to described the separation between adjacent statistically different between adjacent classes for bicycling and classes, based on performance, in UCI road race events for tricycling. -

Tokyo 2020 Paralympic Games Medal Events and Athlete Quotas
Tokyo 2020 Paralympic Games Medal events and athlete quotas 4 September 2017 International Paralympic Committee Adenauerallee 212-214 Tel. +49 228 2097-200 www.paralympic.org 53113 Bonn, Germany Fax +49 228 2097-209 [email protected] Archery • Medal events: 9 (3 men’s, 3 women’s and 3 mixed) • Athlete slots: 140 (80 men and 60 women) • Archery has same number of medals events and athletes as Rio 2016 and the medal events are unchanged. Men’s events (3) Women’s events (3) Mixed events (3) Individual W1 Individual W1 Team W1 Individual Compound Individual Compound Team Compound Individual Recurve Individual Recurve Team Recurve Tokyo 2020 Paralympic Games medal event programme and athlete quotas 2 Athletics • Medal events: 168 (93 men’s, 74 women’s and 1 mixed) • Athlete slots: 1,100 (660 men and 440 women) • The sport will have nine less medal events than Rio 2016 but the same number of athletes • The sport will publish it detailed medal event programme in 2018. Men’s events (93) Women’s events (74) Mixed events (1) To be published in 2018 To be published in 2018 To be published in 2018 Tokyo 2020 Paralympic Games medal event programme and athlete quotas 3 Badminton • Medal events: 14 (7 men’s, 6 women’s and 1 mixed) • Athlete slots: 90 (44 men and 46 women) • Badminton will make its debut at Tokyo 2020. Men’s events (7) Women’s events (6) Mixed events (1) Singles WH1 Singles WH1 Doubles SL/SU Singles WH2 Singles WH2 Singles SL3 Singles SL4 Singles SL4 Singles SU5 Singles SU5 Doubles WH Singles SS6 Doubles SL/SU Doubles WH Tokyo 2020 Paralympic Games medal event programme and athlete quotas 4 Boccia • Medal events: 7 (7 mixed) • Athlete slots: 116 (0 men, 32 women and 84 gender free) • The sport has the same number of medal events as Rio 2016 but 8 more athlete slots as part of the IPC’s aspiration to increase the number of competition opportunities for athletes with high support needs. -

Facilities Environmental Severity Classification Study Report
Facilities Environmental Severity Classification Study Final Report Nicholas A. Silver and Wolfgang Gaebel 16 February 2017 Abstract: This study provides an in-depth review and analysis of existing environmental severity classification and corrosion monitoring efforts and makes recommendations for potentially employing environmental severity classification methodology. By utilizing weather and corrosion data and mapping technology, a baseline environmental severity can be obtained to help decision makers better understand their operating environment and manage the risks associated with corrosion. Executive Summary The Department of Defense (DoD) designs, operates, and manages a vast array of assets, including facilities (buildings, structures, and linear structures), in a variety of environments with varying levels of corrosivity, making corrosion control and sustainment an on-going engineering challenge. The annual cost of corrosion for DoD facilities is estimated at almost $3 billion1. To address corrosion, planning, design, construction, and sustainment personnel must make decisions based on their respective operational requirements and resource availability. In accordance with DoDI 5000.672, the DoD has a requirement to implement Corrosion Protection and Control (CPC) throughout the life cycle of all facilities. Employing environmental severity classification methodology could aid in managing the risks associated with corrosion. Characterization of environmental severity is a technical characteristic that provides a basis for making -

The Paralympic Athlete Dedicated to the Memory of Trevor Williams Who Inspired the Editors in 1997 to Write This Book
This page intentionally left blank Handbook of Sports Medicine and Science The Paralympic Athlete Dedicated to the memory of Trevor Williams who inspired the editors in 1997 to write this book. Handbook of Sports Medicine and Science The Paralympic Athlete AN IOC MEDICAL COMMISSION PUBLICATION EDITED BY Yves C. Vanlandewijck PhD, PT Full professor at the Katholieke Universiteit Leuven Faculty of Kinesiology and Rehabilitation Sciences Department of Rehabilitation Sciences Leuven, Belgium Walter R. Thompson PhD Regents Professor Kinesiology and Health (College of Education) Nutrition (College of Health and Human Sciences) Georgia State University Atlanta, GA USA This edition fi rst published 2011 © 2011 International Olympic Committee Blackwell Publishing was acquired by John Wiley & Sons in February 2007. Blackwell’s publishing program has been merged with Wiley’s global Scientifi c, Technical and Medical business to form Wiley-Blackwell. Registered offi ce: John Wiley & Sons, Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Editorial offi ces: 9600 Garsington Road, Oxford, OX4 2DQ, UK The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK 111 River Street, Hoboken, NJ 07030-5774, USA For details of our global editorial offi ces, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley-blackwell The right of the author to be identifi ed as the author of this work has been asserted in accordance with the UK Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher.