The Enigmatic Processing and Secretion of Interleukin-33
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From IL-15 to IL-33: the Never-Ending List of New Players in Inflammation
From IL-15 to IL-33: the never-ending list of new players in inflammation. Is it time to forget the humble Aspirin and move ahead? Fulvio d’Acquisto, Francesco Maione, Magali Pederzoli-Ribeil To cite this version: Fulvio d’Acquisto, Francesco Maione, Magali Pederzoli-Ribeil. From IL-15 to IL-33: the never-ending list of new players in inflammation. Is it time to forget the humble Aspirin and move ahead?. Bio- chemical Pharmacology, Elsevier, 2009, 79 (4), pp.525. 10.1016/j.bcp.2009.09.015. hal-00544816 HAL Id: hal-00544816 https://hal.archives-ouvertes.fr/hal-00544816 Submitted on 9 Dec 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript Title: From IL-15 to IL-33: the never-ending list of new players in inflammation. Is it time to forget the humble Aspirin and move ahead? Authors: Fulvio D’Acquisto, Francesco Maione, Magali Pederzoli-Ribeil PII: S0006-2952(09)00769-2 DOI: doi:10.1016/j.bcp.2009.09.015 Reference: BCP 10329 To appear in: BCP Received date: 30-7-2009 Revised date: 9-9-2009 Accepted date: 10-9-2009 Please cite this article as: D’Acquisto F, Maione F, Pederzoli-Ribeil M, From IL- 15 to IL-33: the never-ending list of new players in inflammation. -

The IL-1-Like Cytokine IL-33 Is Inactivated After Maturation by Caspase-1
The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1 Corinne Cayrola,b and Jean-Philippe Girarda,b,1 aCentre National de la Recherche Scientifique, Institut de Pharmacologie et de Biologie Structurale, F-31077 Toulouse, France; and bUniversite´de Toulouse, Universite´Paul Sabatier, Institut de Pharmacologie et de Biologie Structurale, F-31077 Toulouse, France Edited by Charles A. Dinarello, University of Colorado Health Sciences Center, Denver, CO, and approved April 10, 2009 (received for review December 13, 2008) IL-33 is a chromatin-associated cytokine of the IL-1 family that has that nuclear IL-33 possesses transcriptional regulatory proper- recently been linked to many diseases, including asthma, rheuma- ties and associates with chromatin in vivo (5). Recently, we found toid arthritis, atherosclerosis, and cardiovascular diseases. IL-33 that IL-33 mimics Kaposi sarcoma herpesvirus for attachment to signals through the IL-1 receptor-related protein ST2 and drives chromatin, and docks, through a short chromatin-binding pep- production of pro-inflammatory and T helper type 2-associated tide, into the acidic pocket formed by the histone H2A-H2B cytokines in mast cells, T helper type 2 lymphocytes, basophils, dimer at the surface of the nucleosome (22). Together, our eosinophils, invariant natural killer T cells, and natural killer cells. findings suggested IL-33 is a dual-function protein that may play It is currently believed that IL-33, like IL-1 and IL-18, requires important roles as both a cytokine and an intracellular nuclear processing by caspase-1 to a mature form (IL-33112–270) for biolog- factor (5, 22). -

Interleukin-33 Signaling Exacerbates Experimental Infectious Colitis by Enhancing Gut Permeability and Inhibiting Protective Th17 Immunity
www.nature.com/mi ARTICLE OPEN Interleukin-33 signaling exacerbates experimental infectious colitis by enhancing gut permeability and inhibiting protective Th17 immunity Vittoria Palmieri1, Jana-Fabienne Ebel1, Nhi Ngo Thi Phuong1, Robert Klopfleisch2, Vivian Pham Vu3,4, Alexandra Adamczyk1, Julia Zöller1, Christian Riedel5, Jan Buer1, Philippe Krebs3, Wiebke Hansen1, Eva Pastille1 and Astrid M. Westendorf 1 A wide range of microbial pathogens is capable of entering the gastrointestinal tract, causing infectious diarrhea and colitis. A finely tuned balance between different cytokines is necessary to eradicate the microbial threat and to avoid infection complications. The current study identified IL-33 as a critical regulator of the immune response to the enteric pathogen Citrobacter rodentium.We observed that deficiency of the IL-33 signaling pathway attenuates bacterial-induced colitis. Conversely, boosting this pathway strongly aggravates the inflammatory response and makes the mice prone to systemic infection. Mechanistically, IL-33 mediates its detrimental effect by enhancing gut permeability and by limiting the induction of protective T helper 17 cells at the site of infection, thus impairing host defense mechanisms against the enteric pathogen. Importantly, IL-33-treated infected mice supplemented with IL-17A are able to resist the otherwise strong systemic spreading of the pathogen. These findings reveal a novel IL-33/IL-17A crosstalk that controls the pathogenesis of Citrobacter rodentium-driven infectious colitis. Manipulating the dynamics of cytokines may offer new therapeutic strategies to treat specific intestinal infections. 1234567890();,: Mucosal Immunology (2021) 14:923–936; https://doi.org/10.1038/s41385-021-00386-7 INTRODUCTION epithelial cells, and endothelial cells in response to injury6. -

Interleukin-18 in Health and Disease
International Journal of Molecular Sciences Review Interleukin-18 in Health and Disease Koubun Yasuda 1 , Kenji Nakanishi 1,* and Hiroko Tsutsui 2 1 Department of Immunology, Hyogo College of Medicine, 1-1 Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan; [email protected] 2 Department of Surgery, Hyogo College of Medicine, 1-1 Mukogawa-cho, Nishinomiya, Hyogo 663-8501, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-798-45-6573 Received: 21 December 2018; Accepted: 29 January 2019; Published: 2 February 2019 Abstract: Interleukin (IL)-18 was originally discovered as a factor that enhanced IFN-γ production from anti-CD3-stimulated Th1 cells, especially in the presence of IL-12. Upon stimulation with Ag plus IL-12, naïve T cells develop into IL-18 receptor (IL-18R) expressing Th1 cells, which increase IFN-γ production in response to IL-18 stimulation. Therefore, IL-12 is a commitment factor that induces the development of Th1 cells. In contrast, IL-18 is a proinflammatory cytokine that facilitates type 1 responses. However, IL-18 without IL-12 but with IL-2, stimulates NK cells, CD4+ NKT cells, and established Th1 cells, to produce IL-3, IL-9, and IL-13. Furthermore, together with IL-3, IL-18 stimulates mast cells and basophils to produce IL-4, IL-13, and chemical mediators such as histamine. Therefore, IL-18 is a cytokine that stimulates various cell types and has pleiotropic functions. IL-18 is a member of the IL-1 family of cytokines. IL-18 demonstrates a unique function by binding to a specific receptor expressed on various types of cells. -

New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets
International Journal of Molecular Sciences Review New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets Jolanta Klonowska 1,* , Jolanta Gle ´n 2, Roman J. Nowicki 2 and Magdalena Trzeciak 2 1 Military Specialist Clinic, Allergy Clinic, ul. D ˛abrowskiego 1, 87-100 Toru´n,Poland 2 Department of Dermatology, Venereology and Allergology Medical University of Gdansk, ul. Kliniczna 1a, 80-401 Gda´nsk,Poland; [email protected] (J.G.); [email protected] (R.J.N.); [email protected] (M.T.) * Correspondence: [email protected]; Tel.: +48-56-622-74-32 Received: 10 September 2018; Accepted: 2 October 2018; Published: 9 October 2018 Abstract: Atopic dermatitis (AD) is a recurrent, chronic, and inflammatory skin disease, which processes with severe itchiness. It often coexists with different atopic diseases. The number of people suffering from AD is relatively high. Epidemiological research demonstrates that 15–30% of children and 2–10% adults suffer from AD. The disease has significant negative social and economic impacts, substantially decreasing the quality of life of the patients and their families. Thanks to enormous progress in science and technology, it becomes possible to recognise complex genetic, immunological, and environmental factors and epidermal barrier defects that play a role in the pathogenesis of AD. We hope that the new insight on cytokines in AD will lead to new, individualised therapy and will open different therapeutic possibilities. In this article, we will focus on the cytokines, interleukin (IL)-17, IL-19, IL-33, and TSLP (thymic stromal lymphopoietin), which play a significant role in AD pathogenesis and may become the targets for future biologic therapies in AD. -

Modulation of the IL-33/IL-13 Axis in Obesity by IL-13Rα2
Modulation of the IL-33/IL-13 Axis in Obesity by IL-13R α2 Jennifer Duffen, Melvin Zhang, Katherine Masek-Hammerman, Angela Nunez, Agnes Brennan, Jessica This information is current as E. C. Jones, Jeffrey Morin, Karl Nocka and Marion Kasaian of September 27, 2021. J Immunol 2018; 200:1347-1359; Prepublished online 5 January 2018; doi: 10.4049/jimmunol.1701256 http://www.jimmunol.org/content/200/4/1347 Downloaded from Supplementary http://www.jimmunol.org/content/suppl/2018/01/05/jimmunol.170125 Material 6.DCSupplemental http://www.jimmunol.org/ References This article cites 64 articles, 22 of which you can access for free at: http://www.jimmunol.org/content/200/4/1347.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 27, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2018 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Modulation of the IL-33/IL-13 Axis in Obesity by IL-13Ra2 Jennifer Duffen,* Melvin Zhang,* Katherine Masek-Hammerman,† Angela Nunez,‡ Agnes Brennan,* Jessica E. -

An Intracrine View of Sex Steroids, Immunity, and Metabolic Regulation
Review An intracrine view of sex steroids, immunity, and metabolic regulation Katya B. Rubinow ABSTRACT Background: Over the past two decades, parallel recognition has grown of the importance of both sex steroids and immune activity in metabolic regulation. More recently, these discrete areas have been integrated in studies examining the metabolic effects of sex steroid immunomodulation. Implicit in these studies has been a traditional, endocrine model of sex steroid delivery from the gonads to target cells, including immune cells. Thus, research to date has focused on the metabolic effects of sex steroid receptor signaling in immune cells. This endocrine model, however, overlooks the extensive capacity of immune cells to generate and metabolize sex steroids, enabling the production of sex steroids for intracrine signaling e that is, sex steroid production for signaling within the cell of origin. Intracrine function allows highly cell-autonomous regulation of sex steroid exposure, and sex steroid secretion by immune cells could confer paracrine signaling effects in neighboring cells within metabolic tissues. In this review, immune cell intracrinology will denote sex steroid production within immune cells for either intracrine or paracrine signaling. This intracrine capacity of immune cells has been well established, and prior work has supported its importance in autoimmune disorders, trauma, and cancer. The potential relevance of immune cell intracrine function to the regulation of energy balance, body weight, body composition, and insulin sensitivity has yet to be explored. Scope of review: The following review will detail findings to date regarding the steroidogenic and steroid metabolizing capacity of immune cells, the regulation of immune cell intracrine function, and the biological effects of immune-derived sex steroids, including the clinical relevance of immune cell intracrinology in fields other than metabolism. -

Sulfation Through the Looking Glass—Recent Advances in Sulfotransferase Research for the Curious
The Pharmacogenomics Journal (2002) 2, 297–308 & 2002 Nature Publishing Group All rights reserved 1470-269X/02 $25.00 www.nature.com/tpj REVIEW Sulfation through the looking glass—recent advances in sulfotransferase research for the curious MWH Coughtrie ABSTRACT Members of the cytosolic sulfotransferase (SULT) superfamily catalyse the Department of Molecular & Cellular Pathology, sulfation of a multitude of xenobiotics, hormones and neurotransmitters. University of Dundee, Ninewells Hospital & Humans have at least 10 functional SULT genes, and a number of recent Medical School, Dundee, Scotland, UK advances reviewed here have furthered our understanding of SULT function. Correspondence: Analysis of expression patterns has shown that sulfotransferases are highly MWH Coughtrie, Department of expressed in the fetus, and SULTs may in fact be a major detoxification Molecular & Cellular Pathology, University enzyme system in the developing human. The X-ray crystal structures of of Dundee, Ninewells Hospital and three SULTs have been solved and combined with mutagenesis experiments Medical School, Dundee DD1 9SY, Scotland, UK. and molecular modelling, they have provided the first clues as to the factors Tel: +44 (0)1382 632510 that govern the unique substrate specificities of some of these enzymes. In Fax: +44 (0)1382 640320 the future these and other studies will facilitate prediction of the fate of E-mail: [email protected] chemicals metabolised by sulfation. Variation in sulfation capacity may be important in determining an individual’s response to xenobiotics, and there has been an explosion in information on sulfotransferase polymorphisms and their functional consequences, including the influence of SULT1A1 genotype on susceptibility to colorectal and breast cancer. -
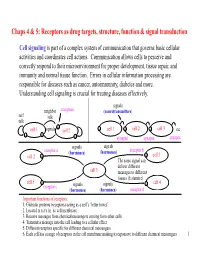
Chaps 4 & 5: Receptors As Drug Targets, Structure, Function & Signal Transduction
Chaps 4 & 5: Receptors as drug targets, structure, function & signal transduction Cell signaling is part of a complex system of communication that governs basic cellular activities and coordinates cell actions. Communication allows cells to perceive and correctly respond to their microenvironment for proper development, tissue repair, and immunity and normal tissue function. Errors in cellular information processing are responsible for diseases such as cancer, autoimmunity, diabetes and more. Understanding cell signaling is crucial for treating diseases effectively. signals neighbor receptors (neurotransmitters) self talk talk cell 1 signals cell 2 cell 1 cell 2 cell 3 etc. synapse synapse synapse signals signals receptor a (hormones) (hormones) receptor b cell 2 cell 3 The same signal can deliver different cell 1 messages to different tissues (histamine). cell 5 cell 4 receptor c signals signals (hormones) (hormones) receptor d Important functions of receptors: 1. Globular proteins (receptors) acting as a cell’s ‘letter boxes’ 2. Located mostly in the cell membrane 3. Receive messages from chemical messengers coming from other cells 4. Transmit a message into the cell leading to a cellular effect 5. Different receptors specific for different chemical messengers 6. Each cell has a range of receptors in the cell membrane making it responsive to different chemical messengers 1 © Oxford University Press, 2013 Signals can be divided into the 5 catagories below. Signal carriers (S) have to reach the proper receptors (R) for the message to be recieved. Intracrine signals are produced by the target cell that stay within the target cell. cell a S = signal S R R = receptor Autocrine signals are produced by the target cell, are secreted, and affect the target cell itself via receptors. -

Evolutionary Divergence and Functions of the Human Interleukin (IL) Gene Family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W
UPDATE ON GENE COMPLETIONS AND ANNOTATIONS Evolutionary divergence and functions of the human interleukin (IL) gene family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W. Nebert3* and Vasilis Vasiliou1 1Molecular Toxicology and Environmental Health Sciences Program, Department of Pharmaceutical Sciences, University of Colorado Denver, Aurora, CO 80045, USA 2Department of Clinical Pharmacy, University of Colorado Denver, Aurora, CO 80045, USA 3Department of Environmental Health and Center for Environmental Genetics (CEG), University of Cincinnati Medical Center, Cincinnati, OH 45267–0056, USA *Correspondence to: Tel: þ1 513 821 4664; Fax: þ1 513 558 0925; E-mail: [email protected]; [email protected] Date received (in revised form): 22nd September 2010 Abstract Cytokines play a very important role in nearly all aspects of inflammation and immunity. The term ‘interleukin’ (IL) has been used to describe a group of cytokines with complex immunomodulatory functions — including cell proliferation, maturation, migration and adhesion. These cytokines also play an important role in immune cell differentiation and activation. Determining the exact function of a particular cytokine is complicated by the influence of the producing cell type, the responding cell type and the phase of the immune response. ILs can also have pro- and anti-inflammatory effects, further complicating their characterisation. These molecules are under constant pressure to evolve due to continual competition between the host’s immune system and infecting organisms; as such, ILs have undergone significant evolution. This has resulted in little amino acid conservation between orthologous proteins, which further complicates the gene family organisation. Within the literature there are a number of overlapping nomenclature and classification systems derived from biological function, receptor-binding properties and originating cell type. -

The IL-33-ILC2 Pathway Protects from Amebic Colitis
bioRxiv preprint doi: https://doi.org/10.1101/2021.06.14.448450; this version posted June 15, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The IL-33-ILC2 pathway protects from amebic colitis 2 Md Jashim Uddin1,4, Jhansi L. Leslie1, Stacey L. Burgess1, Noah Oakland1, Brandon Thompson2, Mayuresh 3 Abhyankar1, Alyse Frisbee2, Alexandra N Donlan2, Pankaj Kumar3, William A Petri, Jr1,2,4# 4 1Department of Medicine: Infectious Diseases and International Health, University of Virginia School of 5 Medicine, Charlottesville, VA, USA 6 2Department of Microbiology, Immunology and Cancer Biology, University of Virginia School of Medicine, 7 Charlottesville, VA, USA 8 3Department of Biochemistry and Molecular Genetics, University of Virginia School of Medicine, 9 Charlottesville, Virginia, USA, 10 4Department of Pathology, University of Virginia School of Medicine, Charlottesville, VA, USA 11 # Address correspondence to: 12 William A. Petri, Jr., M.D., Ph.D. 13 Division of Infectious Diseases and International Health 14 Department of Medicine, University of Virginia 15 Charlottesville, Virginia, 22908-1340 USA 16 Email: [email protected] 17 Tel: (001) 434/924-5621 18 Fax: (001) 434/924-0075 19 The authors have no conflict of interest to declare. 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.06.14.448450; this version posted June 15, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 20 Abstract 21 Entamoeba histolytica is a pathogenic protozoan parasite that causes intestinal colitis, diarrhea, and in 22 some cases, liver abscess. -

Cell Plasticity and Prostate Cancer: the Role of Epithelial–Mesenchymal Transition in Tumor Progression, Invasion, Metastasis and Cancer Therapy Resistance
cancers Review Cell Plasticity and Prostate Cancer: The Role of Epithelial–Mesenchymal Transition in Tumor Progression, Invasion, Metastasis and Cancer Therapy Resistance Sofia Papanikolaou, Aikaterini Vourda, Spyros Syggelos and Kostis Gyftopoulos * Department of Anatomy, University of Patras School of Medicine, Rion, 26504 Patras, Greece; [email protected] (S.P.); [email protected] (A.V.); [email protected] (S.S.) * Correspondence: [email protected] Simple Summary: Although epithelial-to-mesenchymal transition (EMT) is a well-known cellular process involved during normal embryogenesis and wound healing, it also has a dark side; it is a complex process that provides tumor cells with a more aggressive phenotype, facilitating tumor metastasis and even resistance to therapy. This review focuses on the key pathways of EMT in the pathogenesis of prostate cancer and the development of metastases and evasion of currently available treatments. Abstract: Prostate cancer, the second most common malignancy in men, is characterized by high heterogeneity that poses several therapeutic challenges. Epithelial–mesenchymal transition (EMT) is a dynamic, reversible cellular process which is essential in normal embryonic morphogenesis and Citation: Papanikolaou, S.; Vourda, wound healing. However, the cellular changes that are induced by EMT suggest that it may also A.; Syggelos, S.; Gyftopoulos, K. Cell play a central role in tumor progression, invasion, metastasis, and resistance to current therapeutic Plasticity and Prostate Cancer: The options. These changes include enhanced motility and loss of cell–cell adhesion that form a more Role of Epithelial–Mesenchymal aggressive cellular phenotype. Moreover, the reverse process (MET) is a necessary element of the Transition in Tumor Progression, Invasion, Metastasis and Cancer metastatic tumor process.