Inverted Follicular Keratosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary for Narrative Writing
Periodontal Assessment and Treatment Planning Gingival description Color: o pink o erythematous o cyanotic o racial pigmentation o metallic pigmentation o uniformity Contour: o recession o clefts o enlarged papillae o cratered papillae o blunted papillae o highly rolled o bulbous o knife-edged o scalloped o stippled Consistency: o firm o edematous o hyperplastic o fibrotic Band of gingiva: o amount o quality o location o treatability Bleeding tendency: o sulcus base, lining o gingival margins Suppuration Sinus tract formation Pocket depths Pseudopockets Frena Pain Other pathology Dental Description Defective restorations: o overhangs o open contacts o poor contours Fractured cusps 1 ww.links2success.biz [email protected] 914-303-6464 Caries Deposits: o Type . plaque . calculus . stain . matera alba o Location . supragingival . subgingival o Severity . mild . moderate . severe Wear facets Percussion sensitivity Tooth vitality Attrition, erosion, abrasion Occlusal plane level Occlusion findings Furcations Mobility Fremitus Radiographic findings Film dates Crown:root ratio Amount of bone loss o horizontal; vertical o localized; generalized Root length and shape Overhangs Bulbous crowns Fenestrations Dehiscences Tooth resorption Retained root tips Impacted teeth Root proximities Tilted teeth Radiolucencies/opacities Etiologic factors Local: o plaque o calculus o overhangs 2 ww.links2success.biz [email protected] 914-303-6464 o orthodontic apparatus o open margins o open contacts o improper -

Seborrheic Keratosis
Benign Epidermal and Dermal Tumors REAGAN ANDERSON, DO- PROGRAM DIRECTOR, COLORADO DERMATOLOGY INSTITUTE, RVU PGY3 RESIDENTS- JONATHAN BIELFIELD, GEORGE BRANT PGY2 RESIDENT- MICHELLE ELWAY Seborrheic Keratosis Common benign growth seen after third/fourth decade of life Ubiquitous among older individuals Tan to black, macular, papular, or verrucous lesion Occur everywhere except palms, soles, and mucous membranes Can simulate melanocytic neoplasms Pathogenesis: Sun exposure- Australian study found higher incidence in the head/neck Alteration in distribution of epidermal growth factors Somatic activating mutations in fibroblast growth factor receptor and phosphoinositide-3-kinase Seborrheic Keratosis Sign of Leser-Trelat: Rare cutaneous marker of internal malignancy • Gastric/colonic adenocarcinoma, breast carcinoma, and lymphoma m/c • Abrupt increase in number/size of SKs that can occur before, during, or after an internal malignancy is detected • 40% pruritus • M/C location is the back • Malignant acanthosis nigricans may also appear in 20% of patients • Should resolve when primary tumor is treated, and reappear with recurrence/mets Seborrheic Keratosis 6 Histologic types Acanthotic Hyperkeratotic Reticulated Irritated Clonal Melanoacanthoma Borst-Jadassohn phenomenon Well-demarcated nests of keratinocytes within the epidermis Seborrheic Keratoses Treatment Reassurance Irritated SKs (itching, catching on clothes, inflamed) Cryotherapy, curettage, shave excision Pulsed CO2, erbium:YAG lasers Electrodessication Flegel -

Reportable Skin Cancers
11/18/2014 Reportable Skin Cancers 2014/2015 FCDS Educational Webcast Series November 20, 2014 Steven Peace, CTR Anatomy and Physiology of the Integumentary System WHO Classification of Neoplasms of the Skin Signs & Symptoms, Prognostic Factors and Tumor Markers CSv02.05 and SSFs, AJCC TNM 7thed, SS2000 Plus…NCCN Treatment Guidelines The Florida Cancer Data System sincerely thanks the Florida Department of Health, the Centers for Disease Control and Prevention National Program of Cancer Registries, and the University of Miami Miller School of Medicine for their support. 2 1 11/18/2014 Anatomy and Physiology of the Integumentary System Skin or Not Skin - Genital and Non-Genital “Skin” Sites Skin Cancer Facts and Figures Risk Factors – Signs and Symptoms Types of Skin Cancers Overview of Melanoma of Skin Staging Criteria for Melanoma of Skin Overview of Merkel Cell Carcinoma of Skin Staging Criteria for Merkel Cell Carcinoma of Skin Overview of Other Reportable Skin Neoplasms Staging Criteria for Other Reportable Skin Neoplasms 3 Source: http://www.healthandbeautyace.com 4 2 11/18/2014 Defensive Barrier protection from sun protection from injury protection from pathogens protection from environment Thermoregulation controls blood flow regulates evaporation controls release of sweat Vitamin D Production Absorption and Secretion Maintain Body Fluids Balance Excrete Waste Products in Sweat Synthesis of Epidermal Lipids (fats and oils) Sensory Perception and Sensation Touch/Feel/Hot/Cold/Pressure/Vibration/Wind 5 -

Keratoacanthoma As a Postoperative Complication of Skin Cancer Excision
DERMATOLOGIC SURGERY Keratoacanthoma as a postoperative complication of skin cancer excision Leonard H. Goldberg, MD, FRCP,a,b Sirunya Silapunt, MD,a Kathleen K. Beyrau, MD,c S. Ray Peterson, MD,a Paul M. Friedman, MD,a and Murad Alam, MDd Houston, Texas, Portage, Wisconsin; and Chicago, Illinois Background: Keratoacanthomas usually occur spontaneously as a single rapidly growing tumor on sun-exposed skin. Multiple keratoacanthomas are rarely seen. Keratoacanthomas may also develop after trauma, laser resurfacing, radiation therapy, and at the donor site after skin grafting. Objective: We report 6 cases of keratoacanthomas that developed in and around healing and healed surgical sites after treatment of skin cancer. These tumors developed 1 to 3 months after surgery and were sometimes multiple. Methods: We performed follow-up examinations of patients’ wounds after the treatment of skin cancer. Histological examination of nodules developing in the margins of healing wound sites and in the scars of healed wound sites after Mohs micrographic surgery revealed keratoacanthomas. Results: The tumors presented as a rapidly growing nodule or nodules, with the typical morphology and pathology of keratoacanthoma. One patient developed multiple keratoacanthomas at surgical and nonsur- gical sites. These nodules were treated by a combination of excision, curettage and electrodesiccation, and oral isotretinoin, 4 mg/d. Conclusion: Keratoacanthoma must be considered in the differential diagnosis of a rapidly growing nodule within or around the surgical site after skin cancer surgery. (J Am Acad Dermatol 2004;50:753-8.) eratoacanthoma (KA) usually occurs spon- of KA that developed in wounds healing by second taneously as a single rapidly growing tumor intention and in scars within 3 months after Mohs K with characteristic morphology on the sun- micrographic surgery (MMS) for squamous cell car- exposed regions of middle-aged or older persons. -

Clear Cell Acanthoma: a Clinical, Dermoscopic and Histological Review
Clear Cell Acanthoma: A Clinical, Dermoscopic and Histological Review John Howard, DO,* Andrei Gherghina, DO, MS,** Jacquiline Habashy, DO, MS,*** Angela Poulos Combs, DO, FAOCD, FAAD,**** Stanley Skopit, DO, MSE, FAOCD, FAAD***** *Dermatology Research Fellow, Larkin Community Hospital, Miami FL **Dermoscopy Fellow, Skin and Cancer Associates, Plantation, FL ***Traditional Rotating Intern, PGY1, Larkin Community Hospital, Miami, FL ****Dermatopathologist, Global Pathology/Aurora Diagnostics, Miami Lakes, FL *****Program Director, Larkin Community Hospital/NSU-COM Dermatology Residency, South Miami, FL Disclosures: None Correspondence: Jacquiline Habashy, DO; [email protected] Abstract Clear cell acanthoma (CCA) is an uncommon, benign epidermal tumor that may be easily misdiagnosed on a clinical basis alone. Although biopsy is commonly performed for diagnosis, perceptive clinicians may suspect a CCA with the use of clinical and dermoscopic findings. We present a case of a suspected clear cell acanthoma confirmed by biopsy along with a clinical, dermoscopic and histological review of the condition. Introduction arranged in a linear “string of pearls” distribution, of lesions can range from approximately 3 mm to CCA was first described in 1962 and was also revealing the characteristic dermoscopic vascular 20 mm, and they can slowly grow for up to 10 years. pattern seen in clear cell acanthoma (CCA) When closely examining the surface of the lesion, known as “Degos acanthoma” and “acanthome 3,4 cellules claires of Degos and Civatte.”1 There are (Figure 2). vascular puncta are present, which easily bleed currently no known risk factors, and the etiology is following minor trauma. These lesions are usually unknown. It is theorized that the cause may be an Discussion found on the lower extremities in middle-aged to CCA is a rare, benign lesion that is oftentimes elderly adults, with both sexes affected equally.5,6 inflammatory reaction secondary to an unknown difficult to diagnose with clinical observation alone. -
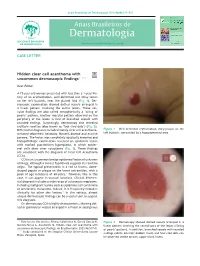
Hidden Clear Cell Acanthoma with Uncommon Dermoscopic Findings
Anais Brasileiros de Dermatologia 2021;96(4):517---525 Anais Brasileiros de Dermatologia www.anaisdedermatologia.org.br CASE LETTER Hidden clear cell acanthoma with ଝ,ଝଝ uncommon dermoscopic findings Dear Editor, A 73-year-old woman presented with less than a 1-year his- tory of an erythematous, well-delimited and shiny lesion on her left buttock, near the gluteal fold (Fig. 1). Der- moscopic examination showed dotted vessels arranged in a linear pattern involving the entire lesion. These vas- cular findings are also called metaphorically a ‘string of pearls’ pattern. Another vascular pattern observed on the periphery of the lesion is that of branched vessels with rounded endings. Surprisingly, dermoscopy also revealed multiple rosettes (also known as ‘four-clod dots’) (Fig. 2). Figure 1 Well delimited erythematous shiny plaque on the Differential diagnoses included mainly clear cell acanthoma, left buttock, surrounded by a hypopigmented area. irritated seborrheic keratosis, Bowen´s disease and eccrine poroma. The lesion was completely surgically removed and histopathologic examination revealed an epidermic lesion with marked psoriasiform hyperplasia, in which epider- mal cells show clear cytoplasms (Fig. 3). These findings are consistent with the diagnosis of Clear Cell Acanthoma (CCA). CCA is an uncommon benign epidermal lesion of unknown etiology, although a recent hypothesis suggests its reactive origin. The typical presentation is a red to brown, dome- shaped papule or plaque on the lower extremities, with a 1 peak of age incidence of 60-years. However, like in this case, it can appear in unusual locations. Clinical differen- tial diagnosis includes a wide range of cutaneous neoplasms, including malignant lesions such as squamous cell carcinoma or amelanotic melanoma. -

Clear Cell Acanthoma: a Review of Clinical and Histologic Variants
Review Clear Cell Acanthoma: A Review of Clinical and Histologic Variants Arif Usmani 1,* and Syeda Qasim 2,* 1 Benchmark Diagnostics, Middleburg Heights, OH 44130, USA 2 School of Graduate Studies, Rutgers University, New Brunswick, NJ 08901, USA * Correspondence: [email protected] (A.U.); [email protected] (S.Q.) Received: 14 July 2020; Accepted: 19 August 2020; Published: 25 August 2020 Abstract: Degos and Civatte first described clear cell acanthoma (CCA) in 1962 and later in a review article found that, in most instances, the lesion was a solitary red-brown dome-shaped papule that involved the distal lower extremity. The first morphologic variant of CCA was reported as a “giant form of the acanthoma of Degos” which measured 45 40 mm, about twice the size of the largest × CCA documented earlier. Since then, many variants of CCA have been described, including polypoid, pigmented and atypical. Herein, we describe a new variant of CCA and add another example of the polypoid variant to the literature. The new variant exhibits cellular features of trichilemmoma but architecturally differs from it. We also attempt to broaden the list of CCA variants summarized by Tempark and Shwayder by adding ours and a few more examples of CCA. The new variants of CCA include verrucous, linear, subungual and trichilemmal. Keywords: clear cell acanthoma; trichilemmoma; glycogen accumulation; Cowden syndrome 1. Introduction Degos and Civatte first described clear cell acanthoma (CCA) in 1962 [1]. Later in 1970, they summarized the experience of the past eight years in another article [2]. They noted that CCA was not uncommon, as more than 100 cases were added to the literature during that period. -

SNODENT (Systemized Nomenclature of Dentistry)
ANSI/ADA Standard No. 2000.2 Approved by ANSI: December 3, 2018 American National Standard/ American Dental Association Standard No. 2000.2 (2018 Revision) SNODENT (Systemized Nomenclature of Dentistry) 2018 Copyright © 2018 American Dental Association. All rights reserved. Any form of reproduction is strictly prohibited without prior written permission. ADA Standard No. 2000.2 - 2018 AMERICAN NATIONAL STANDARD/AMERICAN DENTAL ASSOCIATION STANDARD NO. 2000.2 FOR SNODENT (SYSTEMIZED NOMENCLATURE OF DENTISTRY) FOREWORD (This Foreword does not form a part of ANSI/ADA Standard No. 2000.2 for SNODENT (Systemized Nomenclature of Dentistry). The ADA SNODENT Canvass Committee has approved ANSI/ADA Standard No. 2000.2 for SNODENT (Systemized Nomenclature of Dentistry). The Committee has representation from all interests in the United States in the development of a standardized clinical terminology for dentistry. The Committee has adopted the standard, showing professional recognition of its usefulness in dentistry, and has forwarded it to the American National Standards Institute with a recommendation that it be approved as an American National Standard. The American National Standards Institute granted approval of ADA Standard No. 2000.2 as an American National Standard on December 3, 2018. A standard electronic health record (EHR) and interoperable national health information infrastructure require the use of uniform health information standards, including a common clinical language. Data must be collected and maintained in a standardized format, using uniform definitions, in order to link data within an EHR system or share health information among systems. The lack of standards has been a key barrier to electronic connectivity in healthcare. Together, standard clinical terminologies and classifications represent a common medical language, allowing clinical data to be effectively utilized and shared among EHR systems. -
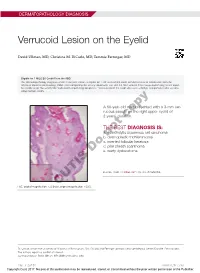
Verrucoid Lesion on the Eyelid
DERMATOPATHOLOGY DIAGNOSIS Verrucoid Lesion on the Eyelid David Ullman, MD; Christina M. DiCarlo, MD; Tammie Ferringer, MD Eligible for 1 MOC SA Credit From the ABD This Dermatopathology Diagnosis article in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatopathology Diagnosis.” You may report the credit after each activity is completed or after accumu- lating multiple credits. A 60-year-old man presented with a 3-mm ver- rucous papule on the right upper eyelid of 2 years’ duration.copy THE BEST DIAGNOSIS IS: a. acantholytic squamous cell carcinoma b. notdesmoplastic trichilemmoma c. inverted follicular keratosis d. pilar sheath acanthoma Doe. warty dyskeratoma PLEASE TURN TO PAGE 227 FOR THE DIAGNOSIS H&E, original magnification ×20 (inset,CUTIS original magnification ×200). Dr. Ullman is from the University of Alabama at Birmingham. Drs. DiCarlo and Ferringer are from Geisinger Medical Center, Danville, Pennsylvania. The authors report no conflict of interest. Correspondence: David Ullman, MD ([email protected]). 216 I CUTIS® WWW.CUTIS.COM Copyright Cutis 2017. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. DERMATOPATHOLOGY DIAGNOSIS DISCUSSION THE DIAGNOSIS: Inverted Follicular Keratosis he differential diagnosis -

Common Skin Tumors
Topic Common Benign epidermal tumors Skin cyst and adnexal neoplasms skin tumors Other common skin tumor Common skin malignancy สมศกดั ิ์ ตนรั ตนากรั 26/02/2015 Seborrheic keratoses Benign Epidermal Tumors very common brown macules, papules, plaques, Seborrheic keratosis or polypoid lesions Dermatosis papulosa nigra over 40 y. Stucco keratosis increase number with age Inverted follicular keratosis Acrokeratosis verruciformis verrucous or 'stuck-on' the Clear cell acanthoma skin Large cell acanthoma predilection for face, neck, Porokeratosis and trunk Epidermal nevus Inflammatory linear verrucous epidermal nevus occur anywhere except Nevus comedonicus mucous membranes, palms, Epidermolytic acanthoma or soles Flegel’s disease sign of Leser-Trélat Cutaneous horn Lichenoid keratosis Acanthosis nigricans Confluent and reticulated papillomatosis Warty dyskeratoma Clinicopathologic Variants Common Seborrheic Keratosis Dermatosis Papulosa Nigra Skin Tags Irritated Seborrheic Keratosis Stucco Keratosis Reticulated Seborrheic Keratosis Clonal Seborrheic Keratoses Seborrheic Keratosis With Squamous Atypia Melanoacanthoma Leser-Trelat sign 1 Dermatosis papulosa nigra Seborrheic keratosis Skin tags Irritated Seborrheic Keratosis Stucco keratoses Epidermal nevi Multiple gray–white keratotic papules on ankle and benign hamartoma of epidermis and dorsal foot. papillary dermis onset usually within the first year of life asymptomatic well-circumscribed, hyperpigmented, papillomatous papules or plaques in a linear -

Dermoscopic Aspect of Verrucous Epidermal Nevi: New Findings
Turkish Journal of Medical Sciences Turk J Med Sci (2019) 49: 710-714 http://journals.tubitak.gov.tr/medical/ © TÜBİTAK Research Article doi:10.3906/sag-1811-27 Dermoscopic aspect of verrucous epidermal nevi: new findings 1, 2 Ömer Faruk ELMAS *, Necmettin AKDENİZ 1 Department of Dermatology and Veneorology, Faculty of Medicine, Ahi Evran University, Kırşehir, Turkey 2 Department of Dermatology and Veneorology, Faculty of Medicine, İstanbul Medeniyet University, İstanbul, Turkey Received: 15.11.2018 Accepted/Published Online: 20.01.2019 Final Version: 18.06.2019 Background/aim: Verrucous epidermal nevi are cutaneous hamartomas with many clinical variants. Dermoscopic features of verrucous epidermal nevus have rarely been investigated. We aimed to identify dermoscopic findings of the entity which will facilitate the diagnostic process by reducing the use of invasive diagnostic methods. Materials and methods: The study included the patients with histopathologically approved verrucous epidermal nevus. Clinical, dermoscopic, and histopathological features of the patients were retrospectively reviewed and the findings identified were recorded. Dermoscopic examination was performed with a polarized-light handheld dermoscope with 10-fold magnification. Results: The most common dermoscopic features were thick brown circles, thick brown branched lines, and terminal hairs. The most common vessel pattern was dotted vessels. Branched thick brown lines, brown globules, brown dots forming lines, serpiginous brown dots, white and brown exophytic papillary structures, fine scale, thick adherent scale, and cerebriform structures were the other findings. Conclusion: We observed many vascular and nonvascular dermoscopic findings which were not described previously for the entity. Dermoscopic examination of the verrucous epidermal nevi may lead to more reliable clinical interpretation and thus may reduce the need for histopathological investigation. -

Squamous Cell Carcinoma Including Actinic Keratosis, Bowen's Disease
Chapter V.4 Squamous Cell Carcinoma Including Actinic Keratosis, Bowen’s Disease, V.4 Keratoacanthoma, and Its Pigmented Variants Iris Zalaudek, Jason S. Giacomel, Bernd Leinweber Contents glected, squamous cell carcinoma may cause local tissue destruction and may metastasize [2, V.4.1 Definition . .295 3]. Moreover, after the diagnosis of an initial V.4.2 Clinical Features . .296 squamous cell carcinoma, the 3-year cumula- V.4.2.1 Invasive Squamous Cell Carcinoma. 296 tive risk for developing a second lesion is ap- V.4.2.2 Actinic Keratosis. .296 proximately 18% [4], emphasizing the need for V.4.2.3 Bowen’s Disease. 297 ongoing clinical surveillance. In its pathogene- V.4.2.4 Keratoacanthoma. .297 sis, chronic ultraviolet (UV) irradiation plays a major role, responsible for DNA mutations (usu- V.4.3 Dermoscopic Criteria. 297 ally in the p53 tumor suppressor gene) in trans- V.4.3.1 Squamous Cell Carcinoma. .297 formed epidermal keratinocytes [5]. This ex- V.4.3.2 Actinic Keratosis. .298 plains why squamous cell carcinoma typically V.4.3.3 Bowen’s Disease. 298 develops on chronic sun-exposed body sites V.4.3.4 Keratoacanthoma. .299 such as the face or forearms of fair-skinned in- V.4.4 Relevant Clinical Differential dividuals. Besides fair skin phototype, male Diagnosis. 299 gender, and age over 40 years, organ-transplant V.4.5 Histopathology. .299 recipients represent a major risk group for squa- V.4.5.1 Squamous Cell Carcinoma. .299 mous cell carcinoma, having up to a 65-fold in- V.4.5.2 Actinic Keratosis.