Use of Dendritic Cell Receptors As Targets for Enhancing Anti-Cancer Immune Responses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Dendritic Cell Subsets in Intestinal Immunity and Inflammation Tian Sun, Albert Nguyen and Jennifer L
Dendritic Cell Subsets in Intestinal Immunity and Inflammation Tian Sun, Albert Nguyen and Jennifer L. Gommerman This information is current as J Immunol 2020; 204:1075-1083; ; of September 28, 2021. doi: 10.4049/jimmunol.1900710 http://www.jimmunol.org/content/204/5/1075 Downloaded from References This article cites 152 articles, 56 of which you can access for free at: http://www.jimmunol.org/content/204/5/1075.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 28, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2020 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Dendritic Cell Subsets in Intestinal Immunity and Inflammation Tian Sun, Albert Nguyen, and Jennifer L. Gommerman The mammalian intestine is a complex environment shaping the ensuing immune response. In this Brief Review,we that is constantly exposed to Ags derived from food, review the different types of classical DC (cDC) located in the microbiota, and metabolites. -

Identification of a Receptor Required for the Anti-Inflammatory Activity of IVIG
Identification of a receptor required for the INAUGURAL ARTICLE anti-inflammatory activity of IVIG Robert M. Anthonya, Fredrik Wermelinga,b, Mikael C. I. Karlssonb, and Jeffrey V. Ravetcha,1 aThe Laboratory of Molecular Genetics and Immunology, The Rockefeller University, New York, NY 10065; and bDepartment of Medicine, Karolinska Institutet, 171 76 Stockholm, Sweden This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 25, 2006. Contributed by Jeffrey V. Ravetch, October 11, 2008 (sent for review October 1, 2008) The anti-inflammatory activity of intravenous Ig (IVIG) results from patients suffering from autoimmune diseases (3). Monomeric IgG, a minor population of the pooled IgG molecules that contains purified from the serum of thousands of healthy donors (IVIG) is terminal ␣2,6-sialic acid linkages on their Fc-linked glycans. These a commonly administered at high doses (1–2 g/kg) for the treatment anti-inflammatory properties can be recapitulated with a fully of a number of autoimmune diseases, including immune-mediated recombinant preparation of appropriately sialylated IgG Fc frag- thrombocytopenia, chronic inflammatory demyelinating polyneu- ments. We now demonstrate that these sialylated Fcs require a ropathy, Kawasaki Disease and Guillain-Barre syndrome, and is specific C-type lectin, SIGN-R1, (specific ICAM-3 grabbing non- widely used in other autoimmune disorders (4–6). integrin-related 1) expressed on macrophages in the splenic mar- A number of hypotheses have been advanced to explain the ginal zone. Splenectomy, loss of SIGN-R1؉ cells in the splenic paradoxical activity of high dose IgG, and include models that marginal zone, blockade of the carbohydrate recognition domain attribute the activity to the polyclonal binding specificities, encoded (CRD) of SIGN-R1, or genetic deletion of SIGN-R1 abrogated the in the variable domains of the administered antibodies that may anti-inflammatory activity of IVIG or sialylated Fc fragments. -

Dendritic Cell Progenitor Trafficking and Identification and Functional Analyses of Dendritic Cells with Distinct Developmental Origin
Dissertation zum Erwerb des Doctor of Philosophy (Ph.D.) an der Medizinischen Fakultät der Ludwig-Maximilians-Universität zu München Dendritic cell progenitor trafficking and identification and functional analyses of dendritic cells with distinct developmental origin vorgelegt von: Johanna Marie Salvermoser aus: Pfaffenhofen an der Ilm Jahr: 2020 _____________________ First supervisor: Prof. Dr. Barbara Schraml Second supervisor: Prof. Dr. Anne Krug Third supervisor: Prof. Dr. Christian Schulz Fourth supervisor: PD. Dr. rer. nat. Caspar Ohnmacht Dean: Prof. Dr. med. dent. Reinhard Hickel Datum der Verteidigung: 09.03.2020 2 Abstract ABSTRACT Conventional dendritic cells (cDCs), are the major antigen-presenting cell type that bridges the innate and adaptive immune system. DCs are constantly replenished from myeloid bone marrow progenitors which latest stage, pre-cDC, leave the BM, seeds the peripheral tissues and further differentiates into two functionally and developmentally distinct subsets, cDC1 and cDC2. This study aimed to investigate DC development by assessing the trafficking of pre-cDCs and by analyzing the effect of a specific depletion of DC progenitors. The signals that regulate the recruitment of pre-cDCs to different peripheral organs are poorly understood. Therefore, this study aimed to identify pre-cDCs in different peripheral organs and to find differences in expression pattern of trafficking receptors. In this study 39 trafficking receptors have been identified to be expressed on pre-cDCs of the analysed tissues and showed differences in the expression patterns between peripheral organs. These receptors are interesting candidates to further study differences in the recruitment of pre-cDCs to different peripheral tissues This can provide possibilities to influence the recruitment of pre-cDCs in certain diseases, where the replenishment of cDCs is accelerated. -

Mycobacterium Tuberculosis Activation by Human Toll-Like Receptors
Human Toll-Like Receptors Mediate Cellular Activation by Mycobacterium tuberculosis Terry K. Means, Shuyan Wang, Egil Lien, Atsutoshi Yoshimura, Douglas T. Golenbock and Matthew J. Fenton This information is current as of September 27, 2021. J Immunol 1999; 163:3920-3927; ; http://www.jimmunol.org/content/163/7/3920 Downloaded from References This article cites 32 articles, 17 of which you can access for free at: http://www.jimmunol.org/content/163/7/3920.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 27, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 1999 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Human Toll-Like Receptors Mediate Cellular Activation by Mycobacterium tuberculosis1 Terry K. Means,* Shuyan Wang,* Egil Lien,† Atsutoshi Yoshimura,† Douglas T. Golenbock,† and Matthew J. Fenton2* Recent studies have implicated a family of mammalian Toll-like receptors (TLR) in the activation of macrophages by Gram- negative and Gram-positive bacterial products. -

Assessing the Tumor Microenvironment by Recovery of Immune Receptor V(D)J Recombination Reads from Tumor Specimen Exome Files
Assessing the Tumor Microenvironment by Recovery of Immune Receptor V(D)J Recombination Reads from Tumor Specimen Exome Files George Blanck, Ph.D. Professor, Molecular Medicine Morsani College of Medicine, USF Immunology Program, Moffitt Cancer Center (not for publication or public web page) Learning objectives: 1. To appreciate the availability of T-cell receptor recombination information from tumor specimen DNA samples. 2. To understand the correlation between T-cell receptor recombinations, in tumor specimen DNA, and other, clinically relevant information for bladder cancer and kidney renal cell carcinoma. 3. To understand the importance of HLA alleles in assessing the impact of T-cell receptor recombinations from tumor specimen DNA. Fig. 1. Immune receptor genes recombine during development to generate many different receptor molecules among the B-cells and T- cells, throughout the body, through life, to bind many different antigens, including tumor antigens. Seven immune receptor genes total: • Three related to antibodies, not further discussed. • Two related to gamma-delta T-cells, not further discussed • Two required for alpha-beta T-cells, the subject of this presentation. Alpha-beta T-cells: • Most numerous. • Best understood, medically speaking. • Generally target peptide antigen, in the case of tumors, a mutant peptide. • The TRB part of the TRA/TRB receptor is considered most important in antigen binding. The tumor specimen and the exome • Surgically remove tumor, obtain DNA sequence (all exons = exome), for tumor mutations, which can guide therapy. • Other cells in the specimen, particularly T-cells. • The DNA representing the T-cell receptor can be identified above the tumor DNA “background”. Fig. -

Macrophage Activation Markers, CD163 and CD206, in Acute-On-Chronic Liver Failure
cells Review Macrophage Activation Markers, CD163 and CD206, in Acute-on-Chronic Liver Failure Marlene Christina Nielsen 1 , Rasmus Hvidbjerg Gantzel 2 , Joan Clària 3,4 , Jonel Trebicka 3,5 , Holger Jon Møller 1 and Henning Grønbæk 2,* 1 Department of Clinical Biochemistry, Aarhus University Hospital, 8200 Aarhus N, Denmark; [email protected] (M.C.N.); [email protected] (H.J.M.) 2 Department of Hepatology & Gastroenterology, Aarhus University Hospital, 8200 Aarhus N, Denmark; [email protected] 3 European Foundation for the Study of Chronic Liver Failure (EF-CLIF), 08021 Barcelona, Spain; [email protected] (J.C.); [email protected] (J.T.) 4 Department of Biochemistry and Molecular Genetics, Hospital Clínic-IDIBAPS, 08036 Barcelona, Spain 5 Translational Hepatology, Department of Internal Medicine I, Goethe University Frankfurt, 60323 Frankfurt, Germany * Correspondence: [email protected]; Tel.: +45-21-67-92-81 Received: 1 April 2020; Accepted: 4 May 2020; Published: 9 May 2020 Abstract: Macrophages facilitate essential homeostatic functions e.g., endocytosis, phagocytosis, and signaling during inflammation, and express a variety of scavenger receptors including CD163 and CD206, which are upregulated in response to inflammation. In healthy individuals, soluble forms of CD163 and CD206 are constitutively shed from macrophages, however, during inflammation pathogen- and damage-associated stimuli induce this shedding. Activation of resident liver macrophages viz. Kupffer cells is part of the inflammatory cascade occurring in acute and chronic liver diseases. We here review the existing literature on sCD163 and sCD206 function and shedding, and potential as biomarkers in acute and chronic liver diseases with a particular focus on Acute-on-Chronic Liver Failure (ACLF). -

Targeting Dendritic Cells with Antigen-Delivering Antibodies for Amelioration of Autoimmunity in Animal Models of Multiple Sclerosis and Other Autoimmune Diseases
antibodies Review Targeting Dendritic Cells with Antigen-Delivering Antibodies for Amelioration of Autoimmunity in Animal Models of Multiple Sclerosis and Other Autoimmune Diseases Courtney A. Iberg and Daniel Hawiger * Department of Molecular Microbiology and Immunology, Saint Louis University School of Medicine, Doisy Research Center, 1205 Carr Lane, St. Louis, MO 63104, USA; [email protected] * Correspondence: [email protected] Received: 31 March 2020; Accepted: 30 April 2020; Published: 15 June 2020 Abstract: The specific targeting of dendritic cells (DCs) using antigen-delivering antibodies has been established to be a highly efficient protocol for the induction of tolerance and protection from autoimmune processes in experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS), as well as in some other animal disease models. As the specific mechanisms of such induced tolerance are being investigated, the newly gained insights may also possibly help to design effective treatments for patients. Here we review approaches applied for the amelioration of autoimmunity in animal models based on antibody-mediated targeting of self-antigens to DCs. Further, we discuss relevant mechanisms of immunological tolerance that underlie such approaches, and we also offer some future perspectives for the application of similar methods in certain related disease settings such as transplantation. Keywords: dendritic cells; tolerance; antigen targeting; chimeric antibodies; autoimmunity; multiple sclerosis; diabetes 1. Introduction Over one hundred years ago, Paul Ehrlich coined the term “horror autotoxicus” to define an immune attack against an organism’s healthy tissues [1]. Since then, our knowledge of the complex mechanisms of the immune system as well as our understanding of the pathogenesis of specific autoimmune diseases have grown tremendously. -

In Sickness and in Health: the Immunological Roles of the Lymphatic System
International Journal of Molecular Sciences Review In Sickness and in Health: The Immunological Roles of the Lymphatic System Louise A. Johnson MRC Human Immunology Unit, MRC Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DS, UK; [email protected] Abstract: The lymphatic system plays crucial roles in immunity far beyond those of simply providing conduits for leukocytes and antigens in lymph fluid. Endothelial cells within this vasculature are dis- tinct and highly specialized to perform roles based upon their location. Afferent lymphatic capillaries have unique intercellular junctions for efficient uptake of fluid and macromolecules, while expressing chemotactic and adhesion molecules that permit selective trafficking of specific immune cell subsets. Moreover, in response to events within peripheral tissue such as inflammation or infection, soluble factors from lymphatic endothelial cells exert “remote control” to modulate leukocyte migration across high endothelial venules from the blood to lymph nodes draining the tissue. These immune hubs are highly organized and perfectly arrayed to survey antigens from peripheral tissue while optimizing encounters between antigen-presenting cells and cognate lymphocytes. Furthermore, subsets of lymphatic endothelial cells exhibit differences in gene expression relating to specific func- tions and locality within the lymph node, facilitating both innate and acquired immune responses through antigen presentation, lymph node remodeling and regulation of leukocyte entry and exit. This review details the immune cell subsets in afferent and efferent lymph, and explores the mech- anisms by which endothelial cells of the lymphatic system regulate such trafficking, for immune surveillance and tolerance during steady-state conditions, and in response to infection, acute and Citation: Johnson, L.A. -

Transcriptional Control of Tissue-Resident Memory T Cell Generation
Transcriptional control of tissue-resident memory T cell generation Filip Cvetkovski Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2019 © 2019 Filip Cvetkovski All rights reserved ABSTRACT Transcriptional control of tissue-resident memory T cell generation Filip Cvetkovski Tissue-resident memory T cells (TRM) are a non-circulating subset of memory that are maintained at sites of pathogen entry and mediate optimal protection against reinfection. Lung TRM can be generated in response to respiratory infection or vaccination, however, the molecular pathways involved in CD4+TRM establishment have not been defined. Here, we performed transcriptional profiling of influenza-specific lung CD4+TRM following influenza infection to identify pathways implicated in CD4+TRM generation and homeostasis. Lung CD4+TRM displayed a unique transcriptional profile distinct from spleen memory, including up-regulation of a gene network induced by the transcription factor IRF4, a known regulator of effector T cell differentiation. In addition, the gene expression profile of lung CD4+TRM was enriched in gene sets previously described in tissue-resident regulatory T cells. Up-regulation of immunomodulatory molecules such as CTLA-4, PD-1, and ICOS, suggested a potential regulatory role for CD4+TRM in tissues. Using loss-of-function genetic experiments in mice, we demonstrate that IRF4 is required for the generation of lung-localized pathogen-specific effector CD4+T cells during acute influenza infection. Influenza-specific IRF4−/− T cells failed to fully express CD44, and maintained high levels of CD62L compared to wild type, suggesting a defect in complete differentiation into lung-tropic effector T cells. -

Single-Cell RNA Sequencing Demonstrates the Molecular and Cellular Reprogramming of Metastatic Lung Adenocarcinoma
ARTICLE https://doi.org/10.1038/s41467-020-16164-1 OPEN Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma Nayoung Kim 1,2,3,13, Hong Kwan Kim4,13, Kyungjong Lee 5,13, Yourae Hong 1,6, Jong Ho Cho4, Jung Won Choi7, Jung-Il Lee7, Yeon-Lim Suh8,BoMiKu9, Hye Hyeon Eum 1,2,3, Soyean Choi 1, Yoon-La Choi6,10,11, Je-Gun Joung1, Woong-Yang Park 1,2,6, Hyun Ae Jung12, Jong-Mu Sun12, Se-Hoon Lee12, ✉ ✉ Jin Seok Ahn12, Keunchil Park12, Myung-Ju Ahn 12 & Hae-Ock Lee 1,2,3,6 1234567890():,; Advanced metastatic cancer poses utmost clinical challenges and may present molecular and cellular features distinct from an early-stage cancer. Herein, we present single-cell tran- scriptome profiling of metastatic lung adenocarcinoma, the most prevalent histological lung cancer type diagnosed at stage IV in over 40% of all cases. From 208,506 cells populating the normal tissues or early to metastatic stage cancer in 44 patients, we identify a cancer cell subtype deviating from the normal differentiation trajectory and dominating the metastatic stage. In all stages, the stromal and immune cell dynamics reveal ontological and functional changes that create a pro-tumoral and immunosuppressive microenvironment. Normal resident myeloid cell populations are gradually replaced with monocyte-derived macrophages and dendritic cells, along with T-cell exhaustion. This extensive single-cell analysis enhances our understanding of molecular and cellular dynamics in metastatic lung cancer and reveals potential diagnostic and therapeutic targets in cancer-microenvironment interactions. 1 Samsung Genome Institute, Samsung Medical Center, Seoul 06351, Korea. -
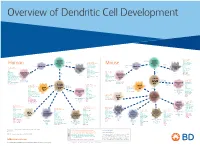
View Dendritic Cell Development Poster
Overview of Dendritic Cell Development Lineage–, CD45+, Common CD117 (c-kit) Common MHCII+, CD11c+ – + CD207 (Langerin) Myeloid CD117 (c-kit) Lineage , CD45 , Myeloid Progenitor MHCII (HLA-DR)+, CD11c+ Progenitor CD324 (E-Cadherin) Human Mouse CD326 (EpCAM) CD207 (Langerin) TGFb1 Cells CD11b, CD115 Cells CD14 Monocyte CD324 (E-Cadherin) Monocyte M-CSF CD11b – + Ly6C Langerhans CD24 Lineage , CD45 , M-CSF CD326 (EpCAM) MHCII (HLA-DR)+, CD11c+ Langerhans CD11blo Zbtb46– Cells CD172a (Sirp-α) CD16 CD1ahi, CD1c CD205 (DEC-205) Cells CSF F4/80 CD64 CD172a (Sirp-α) Lineage–, CD45+, FLT3L TLR3, TLR11 CD1a, CD1c Inflammatory CD369 (Dectin-1/CLEC7A) MHCII+, CD11c+ +/– CSF IL-15 CD8–, CD14– CD11b, CD14 CD371 (CLEC12A) CD64 Monocyte- FLT3L Inflammatory CD370 (Clec9a)– CD172a (Sirp-α) IL-15 CLEC6A CD11b derived lo Monocyte- CD206, CD209 (DC-SIGN) TLR1, TLR2, TLR3 , TLR6 CD209a (DC-SIGN) CD367 (DCIR/CLEC4A) DCs CD14– CD272 (BTLA)lo derived CD369 (Dectin-1/CLEC7A) DCs Common Ly-6C – + CD371 (CLEC12A) CD117 (c-kit) Lineage , CD45 , IL-1β, IL-6, IL-10, TLR1-6, TLR7-8, TLR10 Dendritic + lo CLEC6A – – CD135/FLT3 MHCII , CD11c IL-12, IL-23, TNF CD8a , CD14 IL-1β, IL-6 IL-10, Precursor TLR3lo, TLR4, TLR7, TLR8 CD45R (B220) IL-12, IL-23, TNF Plasmacytoid CD207 (Langerin)– Cells CD317 (BST-2) Common Lineage–, CD45+, FLT3L DCs Lineage–, CD45+, + Ly6C + lo/– CD207 IFN Type I + + Dendritic CD135/FLT3 MHCII (HLA-DR) , CD11c Lineage–, CD45+, IRF7, IRF8, BATF3hi Siglec-H MHCII (HLA-DR) , CD11c hi – + CD123 + + Dermal SpiB, Zbtb46 CD1a, CD64 CD1a Precursor CD117 (c-kit)