Gallwasp Species in Britain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Leafy and Crown Gall
Is it Crown Gall or Leafy Gall? Melodie L. Putnam and Marilyn Miller Humphrey Gifford, an early English poet said, “I cannot say the crow is white, But needs must call a spade a spade.” To call a thing by its simplest and best understood name is what is meant by calling a spade a spade. We have found confusion around the plant disease typified by leafy galls and shoot proliferation, and we want to call a spade a spade. The bacterium Rhodococcus fascians causes fasciation, leafy galls and shoot proliferation on plants. These symptoms have been attributed variously to crown gall bacteria (Agrobacterium tumefaciens), virus infection, herbicide damage, or eriophyid mite infestation. There is also confusion about what to call the Figure 1. Fasciation (flattened growth) of a pumpkin symptoms caused by R. fascians. Shoot stem, which may be due to disease, a genetic proliferation and leafy galls are sometimes condition, or injury. called “fasciation,” a term also used to refer to tissues that grow into a flattened ribbon- like manner (Figure 1). The root for the word fasciation come from the Latin, fascia, to fuse, and refers to a joining of tissues. We will reserve the term fasciation for the ribbon like growth of stems and other organs. The terms “leafy gall” and “shoot proliferation” are unfamiliar to many people, but are a good description of what is seen on affected plants. A leafy gall is a mass of buds or short shoots tightly packed together and fused at the base. These may appear beneath the soil or near the soil line at the base of the stem (Figure 2). -

Checklist of British and Irish Hymenoptera - Cynipoidea
Biodiversity Data Journal 5: e8049 doi: 10.3897/BDJ.5.e8049 Taxonomic Paper Checklist of British and Irish Hymenoptera - Cynipoidea Mattias Forshage‡, Jeremy Bowdrey§, Gavin R. Broad |, Brian M. Spooner¶, Frank van Veen# ‡ Swedish Museum of Natural History, Stockholm, Sweden § Colchester and Ipswich Museums, Colchester, United Kingdom | The Natural History Museum, London, United Kingdom ¶ Royal Botanic Gardens, Kew, Richmond, United Kingdom # University of Exeter, Penryn, United Kingdom Corresponding author: Gavin R. Broad ([email protected]) Academic editor: Pavel Stoev Received: 05 Feb 2016 | Accepted: 06 Mar 2017 | Published: 09 Mar 2017 Citation: Forshage M, Bowdrey J, Broad G, Spooner B, van Veen F (2017) Checklist of British and Irish Hymenoptera - Cynipoidea. Biodiversity Data Journal 5: e8049. https://doi.org/10.3897/BDJ.5.e8049 Abstract Background The British and Irish checklist of Cynipoidea is revised, considerably updating the last complete checklist published in 1978. Disregarding uncertain identifications, 220 species are now known from Britain and Ireland, comprising 91 Cynipidae (including two established non-natives), 127 Figitidae and two Ibaliidae. New information One replacement name is proposed, Kleidotoma thomsoni Forshage, for the secondary homonym Kleidotoma tetratoma Thomson, 1861 (nec K. tetratoma (Hartig, 1841)). © Forshage M et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 2 Forshage M et al Introduction This paper continues the series of updated British and Irish Hymenoptera checklists that started with Broad and Livermore (2014a), Broad and Livermore (2014b), Liston et al. -

Quercus Cerris
Quercus cerris Quercus cerris in Europe: distribution, habitat, usage and threats D. de Rigo, C. M. Enescu, T. Houston Durrant, G. Caudullo Turkey oak (Quercus cerris L.) is a deciduous tree native to southern Europe and Asia Minor, and a dominant species in the mixed forests of the Mediterranean basin. Turkey oak is a representative of section Cerris, a particular section within the genus Quercus which includes species for which the maturation of acorns occurs in the second year. Quercus cerris L., commonly known as Turkey oak, is a large fast-growing deciduous tree species growing to 40 m tall with 1 Frequency a trunk up to 1.5-2 m diameter , with a well-developed root < 25% system2. It can live for around 120-150 years3. The bark is 25% - 50% 50% - 75% mauve-grey and deeply furrowed with reddish-brown or orange > 75% bark fissures4, 5. Compared with other common oak species, e.g. Chorology Native sessile oak (Quercus petraea) and pedunculate oak (Quercus Introduced robur), the wood is inferior, and only useful for rough work such as shuttering or fuelwood1. The leaves are dark green above and grey-felted underneath6; they are variable in size and shape but are normally 9-12 cm long and 3-5 cm wide, with 7-9 pairs of triangular lobes6. The leaves turn yellow to gold in late autumn and drop off or persist in the crown until the next spring, especially on young trees3. The twigs are long and pubescent, grey or olive-green, with lenticels. The buds, which are concentrated Large shade tree in agricultural area near Altamura (Bari, South Italy). -

The Dorset Heath 2013 So Once Again You Have Me As Editor
NewsletterThe ofD theo Dorsetrset Flora H eGroupath 201 4 Chairman and VC9 Recorder Robin Walls; Secretary Laurence Taylor Editorial: John Newbould It would appear that the group had no complaints about the layout and content of the Dorset Heath 2013 so once again you have me as editor. The year was somewhat difficult for me as somehow, whenever I had to leave the room in Yorkshire Naturalists’ Union committee meetings in 2011, they managed to appoint me President for 2013 resulting in extra commitments in that county. During April 2013, Dorset hosted the National Forum for Biological Recording’s annual conference at the R.N.L.I. College at Poole. What a fabulous conference venue and the overnight accommodation was excellent. NFBR then joined Dorset naturalists with a joint meeting based at Studland helping to survey for the Cyril Diver project. Once again, duties took me away as I seem to be the conference administrator. The Flora Group had an interesting year, with variable numbers at field meetings. Never-the-less some important recording has been achieved including members engaging with recording bryophytes for the first time, one meeting to record fungi near Hardy’s Cottage, which thanks to the expertise of Bryan Edwards was very successful. We also had a few members try their hand at lichen recording In June 2014, I have been tasked by the Linnean Society to organise their annual field trip, which will be in June starting with a day on Portland and Chesil on the Saturday with Ballard Down and Studland on the Sunday. -
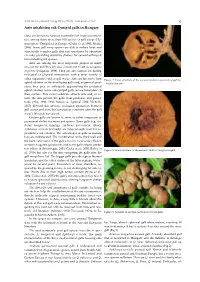
Ants Inhabiting Oak Cynipid Galls in Hungary
North-Western Journal of Zoology 2020, vol.16 (1) - Correspondence: Notes 95 Ants inhabiting oak Cynipid galls in Hungary Oaks are known to harbour extremely rich insect communi- ties, among them more than 100 species of gall wasps (Hy- menoptera: Cynipidae) in Europe (Csóka et al. 2005, Melika 2006). Some gall wasp species are able to induce large and structurally complex galls that can sometimes be abundant on oaks, providing attractive shelters for several arthropod taxa including ant species. Ants are among the most important players in many ecosystems and they are also considered to act as ecosystem engineers (Folgarait, 1998). They are also famous for having ecological or physical interactions with a great variety of other organisms, such as gall wasps. Ants are known to tend Figure 1. Inner structure of the asexual Andricus quercustozae gall in- aphid colonies on the developing galls and, as general pred- habited by ants. ators, they prey on arthropods approaching the protected aphid colonies. Some oak cynipid galls secrete honeydew on their surface. This sweet substrate attracts ants and, in re- turn, the ants protect the galls from predators and parasi- toids (Abe, 1988, 1992; Inouye & Agrawal 2004; Nicholls, 2017). Beyond this obvious ecological interaction between gall wasps and ants, this association continues after the gall wasp’s life cycle has ceased. Certain galls are known to serve as either temporary or permanent shelter for many ant species. Some galls (e.g. An- dricus hungaricus (Hartig), Andricus quercustozae (Bosc), Aphelonyx cerricola (Giraud)) are large enough even for re- productive ant colonies. The advantages of galls as nesting logs are multifaceted. -

Taxonomic Notes and Type Designations of Gall Inducing Cynipid Wasps Described by G.Mayr (Insecta: Hymenoptera: Cynipidae)
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Annalen des Naturhistorischen Museums in Wien Jahr/Year: 2001 Band/Volume: 103B Autor(en)/Author(s): Bechtold M., Melika George Artikel/Article: Taxonomic notes and type designations of gall inducing cynipid wasps described by G.Mayr (Insecta: Hymenoptera: Cynipidae). 327-339 ©Naturhistorisches Museum Wien, download unter www.biologiezentrum.at Ann. Naturhist. Mus. Wien 103 B 327 - 339 Wien, Dezember 2001 Taxonomic notes and type designations of gall inducing cynipid wasps described by G. Mayr (Insecta: Hymenoptera: Cynipidae) G. Melika & M. Bechtold* Abstract Lectotypes for twelve of Mayr's cynipid gall wasp species (Hymenoptera: Cynipidae: Cynipinae) are desi- gnated. From twenty cynipid gall wasp species, described by Mayr, seven have already been synonymized, and thirteen species are still valid. Andricus insana (WESTWOOD, 1837) syn.n. is a new synonym of Andricus quercustozae (Bosc, 1792). Key words: Cynipidae, gall wasps, Hymenoptera, lectotype designation, Gustav Mayr, new synonymy, taxonomy. Zusammenfassung Lectotypen für zwölf der von Mayr beschriebenen Gallwespenarten (Hymenoptera: Cynipidae: Cynipinae) werden designiert. Mayr hat zwanzig Gallwespenarten beschrieben, davon sind sieben bereits synonymi- siert worden, dreizehn Arten sind noch gültig. Andricus insana (WESTWOOD, 1837) syn.n. ist ein neues Synonym von Andricus quercustozae (Bosc, 1792). Introduction Gustav Mayr, a famous Austrian entomologist, described eleven genera of gall inducing Cynipidae and twenty species from twelve genera (Hymenoptera: Cynipoidea). Seven of them have already been synonymized, while thirteen species are still valid. However, he never designated types for his newly described species. All the specimens are syn- or cotypes and usually these specimens were marked with "Type" or even not so. -

The Structure of Cynipid Oak Galls: Patterns in the Evolution of an Extended Phenotype
The structure of cynipid oak galls: patterns in the evolution of an extended phenotype Graham N. Stone1* and James M. Cook2 1Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK ([email protected]) 2Department of Biology, Imperial College, Silwood Park, Ascot, Berkshire SL5 7PY, UK Galls are highly specialized plant tissues whose development is induced by another organism. The most complex and diverse galls are those induced on oak trees by gallwasps (Hymenoptera: Cynipidae: Cyni- pini), each species inducing a characteristic gall structure. Debate continues over the possible adaptive signi¢cance of gall structural traits; some protect the gall inducer from attack by natural enemies, although the adaptive signi¢cance of others remains undemonstrated. Several gall traits are shared by groups of oak gallwasp species. It remains unknown whether shared traits represent (i) limited divergence from a shared ancestral gall form, or (ii) multiple cases of independent evolution. Here we map gall character states onto a molecular phylogeny of the oak cynipid genus Andricus, and demonstrate three features of the evolution of gall structure: (i) closely related species generally induce galls of similar structure; (ii) despite this general pattern, closely related species can induce markedly di¡erent galls; and (iii) several gall traits (the presence of many larval chambers in a single gall structure, surface resins, surface spines and internal air spaces) of demonstrated or suggested adaptive value to the gallwasp have evolved repeatedly. We discuss these results in the light of existing hypotheses on the adaptive signi¢cance of gall structure. Keywords: galls; Cynipidae; enemy-free space; extended phenotype; Andricus layers of woody or spongy tissue, complex air spaces within 1. -

National Oak Gall Wasp Survey
ational Oak Gall Wasp Survey – mapping with parabiologists in Finland Bess Hardwick Table of Contents 1. Introduction ................................................................................................................. 2 1.1. Parabiologists in data collecting ............................................................................. 2 1.2. Oak cynipid gall wasps .......................................................................................... 3 1.3. Motivations and objectives .................................................................................... 4 2. Material and methods ................................................................................................ 5 2.1. The volunteers ........................................................................................................ 5 2.2. Sampling ................................................................................................................. 6 2.3. Processing of samples ............................................................................................ 7 2.4. Data selection ........................................................................................................ 7 2.5. Statistical analyses ................................................................................................. 9 3. Results ....................................................................................................................... 10 3.1. Sampling success ................................................................................................. -

The Population Biology of Oak Gall Wasps (Hymenoptera:Cynipidae)
5 Nov 2001 10:11 AR AR147-21.tex AR147-21.SGM ARv2(2001/05/10) P1: GSR Annu. Rev. Entomol. 2002. 47:633–68 Copyright c 2002 by Annual Reviews. All rights reserved THE POPULATION BIOLOGY OF OAK GALL WASPS (HYMENOPTERA:CYNIPIDAE) Graham N. Stone,1 Karsten Schonrogge,¨ 2 Rachel J. Atkinson,3 David Bellido,4 and Juli Pujade-Villar4 1Institute of Cell, Animal, and Population Biology, University of Edinburgh, The King’s Buildings, West Mains Road, Edinburgh EH9 3JT, United Kingdom; e-mail: [email protected] 2Center of Ecology and Hydrology, CEH Dorset, Winfrith Technology Center, Winfrith Newburgh, Dorchester, Dorset DT2 8ZD, United Kingdom; e-mail: [email protected] 3Center for Conservation Science, Department of Biology, University of Stirling, Stirling FK9 4LA, United Kingdom; e-mail: [email protected] 4Departamento de Biologia Animal, Facultat de Biologia, Universitat de Barcelona, Avenida Diagonal 645, 08028 Barcelona, Spain; e-mail: [email protected] Key Words cyclical parthenogenesis, host alternation, food web, parasitoid, population dynamics ■ Abstract Oak gall wasps (Hymenoptera: Cynipidae, Cynipini) are characterized by possession of complex cyclically parthenogenetic life cycles and the ability to induce a wide diversity of highly complex species- and generation-specific galls on oaks and other Fagaceae. The galls support species-rich, closed communities of inquilines and parasitoids that have become a model system in community ecology. We review recent advances in the ecology of oak cynipids, with particular emphasis on life cycle characteristics and the dynamics of the interactions between host plants, gall wasps, and natural enemies. We assess the importance of gall traits in structuring oak cynipid communities and summarize the evidence for bottom-up and top-down effects across trophic levels. -

Butlleti 71.P65
Butll. Inst. Cat. Hist. Nat., 71: 83-95. 2003 ISSN: 1133-6889 GEA, FLORA ET FAUNA The life cycle of Andricus hispanicus (Hartig, 1856) n. stat., a sibling species of A. kollari (Hartig, 1843) (Hymenoptera: Cynipidae) Juli Pujade-Villar*, Roger Folliot** & David Bellido* Rebut: 28.07.03 Acceptat: 01.12.03 Abstract and so we consider A. mayeti and A. niger to be junior synonyms of A. hispanicus. Finally, possible causes of the speciation of A. kollari and The marble gallwasp, Andricus kollari, common A. hispanicus are discussed. and widespread in the Western Palaeartic, is known for the conspicuous globular galls caused by the asexual generations on the buds of several KEY WORDS: Cynipidae, Andricus, A. kollari, A. oak species. The sexual form known hitherto, hispanicus, biological cycle, sibling species, formerly named Andricus circulans, makes small sexual form, speciation, distribution, morphology, gregarious galls on the buds of Turkey oak, A. mayeti, A. burgundus. Quercus cerris; this oak, however, is absent from the Iberian Peninsula, where on the other hand the cork oak, Q. suber, is present. Recent genetic studies show the presence of two different Resum populations or races with distribution patterns si- milar to those of Q. cerris and Q. suber. We present new biological and morphological Cicle biològic d’Andricus hispanicus (Hartig, evidence supporting the presence of a sibling 1856) una espècie bessona d’A. kollari (Hartig, species of A. kollari in the western part of its 1843) (Hymenoptera: Cynipidae) range (the Iberian Peninsula, southern France and North Africa), Andricus hispanicus n. stat.. Biological and morphological differences separating these Andricus kollari és una espècie molt comuna dis- two species from other closely related ones are tribuida a l’oest del paleartic coneguda per la given and the new sexual form is described for the gal·la globular i relativament gran de la generació first time. -

Fossil Oak Galls Preserve Ancient Multitrophic Interactions
Edinburgh Research Explorer Fossil oak galls preserve ancient multitrophic interactions Citation for published version: Stone, GN, van der Ham, RWJM & Brewer, JG 2008, 'Fossil oak galls preserve ancient multitrophic interactions', Proceedings of the Royal Society B-Biological Sciences, vol. 275, no. 1648, pp. 2213-2219. https://doi.org/10.1098/rspb.2008.0494 Digital Object Identifier (DOI): 10.1098/rspb.2008.0494 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Proceedings of the Royal Society B-Biological Sciences Publisher Rights Statement: Free in PMC. General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 01. Oct. 2021 Proc. R. Soc. B (2008) 275, 2213–2219 doi:10.1098/rspb.2008.0494 Published online 17 June 2008 Fossil oak galls preserve ancient multitrophic interactions Graham N. Stone1,*, Raymond W. J. M. van der Ham2 and Jan G. Brewer3 1Institute of Evolutionary Biology, University of Edinburgh, West Mains Road, Edinburgh EH9 3JT, UK 2Nationaal Herbarium Nederland, Universiteit Leiden, PO Box 9514, 2300 RA Leiden, The Netherlands 3Hogebroeksweg 32, 8102 RK Raalte, The Netherlands Trace fossils of insect feeding have contributed substantially to our understanding of the evolution of insect–plant interactions. -

A New Species of Woody Tuberous Oak Galls from Mexico
Dugesiana 19(2): 79-85 Fecha de publicación: 21 de diciembre 2012 © Universidad de Guadalajara A new species of woody tuberous oak galls from Mexico (Hymenoptera: Cynipidae) and notes with related species Una nueva especie de agalla leñosa tuberosa en encinos de México (Hymenoptera: Cynipidae) y anotaciones sobre las especies relacionadas Juli Pujade-Villar & Jordi Paretas-Martínez Universitat de Barcelona, Facultat de Biologia, Departament de Biologia Animal, Avda. Diagonal 645, 08028-Barcelona (Spain). E-mail: [email protected] (corresponding author). ABSTRACT A new species of cynipid gallwasp, Andricus tumefaciens n. sp. (Hymenoptera: Cynipidae: Cynipini), is described from Mexico. This species induces galls on twigs of Quercus chihuahuensis Trelease, white oaks (Quercus, section Quercus s.s.). Diagnosis, full description, biology and distribution data of Andricus tumefaciens n. sp. are given. Some morphological characters are discussed and illustrated, and compared to related species (A. durangensis Beutenmüller from Mexico and A. wheeleri Beutenmüller from USA). Andricus cameroni Ashmead is considered as ‘nomen nudum’. Key words: Cynipidae, tuberous gall, Andricus, taxonomy, morphology, distribution, biology. RESUMEN Se describe de México una nueva especie de cinípido gallícola de encinos: Andricus tumefaciens n. sp. (Hymenoptera: Cynipidae: Cynipini). Esta especie induce agallas en ramas de una especie de roble blanco: Quercus chihuahuensis Trelease (Quercus, sección Quercus s.s.). Se aporta una diagnosis, la descripción completa, biología y distribución de dicha nueva especie. Se ilustran y discuten los caracteres morfológicos, y se comparan con las especies relacionadas (A. durangensis Beutenmüller de México y A. wheeleri Beutenmüller de EE.UU.). Andricus cameroni Ashmead es considerada como “nomen nudum”. Palabras clave: Cynipidae, agalla tuberosa, Andricus, taxonomía, morfología, distribución, biología.