Minutes of the CHMP Meeting 11-14 November 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Drug Limitations Mary C
RON DESANTIS GOVERNOR SUMMARY OF DRUG LIMITATIONS MARY C. MAYHEW SECRETARY **Medications listed in this document may or may not require a prior authorization. Please view the Preferred Drug List at: http://ahca.myflorida.com/Medicaid/Prescribed_Drug/pharm_thera/fmpdl.shtml** Summary of Drug Limitations Abilify (aripiprazole) 2mg, 5mg, 20mg, 30mg tablets Minimum age = 6; Maximum of 1 tablet per day Abilify (aripiprazole) 10mg, 15mg tablets Minimum age = 6; Maximum of 15mg per day for ages = 6 - 11; Maximum of 30mg per day for ages = 12-17 Maximum of 1 tablet per day Abilify (aripiprazole) Discmelt 10mg, 15mg tabs Minimum age = 6; Maximum of 15mg per day for ages = 6 - 11; Maximum of 30mg per day for ages = 12-17; Maximum of 2 tablets per day Abilify (aripiprazole) 1mg/ml solution Minimum age = 6; Maximum of 15ml per day for ages = 6 - 11; Maximum of 30ml per day for ages = 12-17; Maximum of 30ml per day for ages =/> 18 Abilify Maintena (aripiprazole) syringe/vial Minimum age = 18; Maximum of 1 syringe or vial every 28 days Absorica (isotretinoin) 10mg, 20mg, 25mg,30mg, 35mg, & Minimum age = 12 40mg capsules Abstral (fentanyl citrate) sublingual tablets Minimum age = 18; Maximum of 4 sublingual tablets per day Acanya (benzoyl peroxide/clindamycin)Gel, gel pump Minimum Age= 12 Accolate (zafirlukast) tablets Maximum of 3 tablets per day Aciphex (rabeprazole) 5mg, 10mg sprinkle capsules Minimum age = 1; Maximum age = 11; Maximum of 1 capsule per day Aciphex (rabeprazole) 20mg tablets Minimum age = 1; Maximum of 2 tablets per day Actemra (tocilizumab) 80mg/4ml, 200mg/10ml, Minimum age= 2 400mg/20ml Vials, & 162mg/0.9ml Syringe Actimmune (Interferon Gamma-1b) Maximum of 6ml every 28days Actiq (fentanyl citrate) Lozenges Minimum age = 18; Maximum of 4 lozenges per day Activella (estradiol/norethindrone) tablets Minimum age = 18 Updated 02/28/2019 1 RON DESANTIS GOVERNOR SUMMARY OF DRUG LIMITATIONS MARY C. -

XERMELO™ (Telotristat Ethyl)
PHARMACY COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 5/18/2017 SECTION: DRUGS LAST REVIEW DATE: 5/20/2021 LAST CRITERIA REVISION DATE: 5/20/2021 ARCHIVE DATE: XERMELO™ (telotristat ethyl) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage Guideline must be read in its entirety to determine coverage eligibility, if any. This Pharmacy Coverage Guideline provides information related to coverage determinations only and does not imply that a service or treatment is clinically appropriate or inappropriate. The provider and the member are responsible for all decisions regarding the appropriateness of care. Providers should provide BCBSAZ complete medical rationale when requesting any exceptions to these guidelines. The section identified as “Description” defines or describes a service, procedure, medical device or drug and is in no way intended as a statement of medical necessity and/or coverage. The section identified as “Criteria” defines criteria to determine whether a service, procedure, medical device or drug is considered medically necessary or experimental or investigational. State or federal mandates, e.g., FEP program, may dictate that any drug, device or biological product approved by the U.S. Food and Drug Administration (FDA) may not be considered experimental or investigational and thus the drug, device or biological product may be assessed only on the basis of medical necessity. Pharmacy Coverage Guidelines are subject to change as new information becomes available. For purposes of this Pharmacy Coverage Guideline, the terms "experimental" and "investigational" are considered to be interchangeable. BLUE CROSS®, BLUE SHIELD® and the Cross and Shield Symbols are registered service marks of the Blue Cross and Blue Shield Association, an association of independent Blue Cross and Blue Shield Plans. -

Nye Legemidler Som Ikke Har Markedsføringstillatelse for Legemidler Oppført På Listen Gjelder Retningslinjens Vilkår Uavhengig Av Indikasjon for Bruken
Nye legemidler som ikke har markedsføringstillatelse For legemidler oppført på listen gjelder retningslinjens vilkår uavhengig av indikasjon for bruken. Oppdatert: 13.11.2018 Orphan medicinal Indikasjon per nå. product (* Se (Samt mulig andre indikasjoner i informasjon Oppført på Virkestoff fremtiden.) nederst) listen Depatuximab mafodotin Treatment of glioblastoma (GBM) nov.18 Alpelisib Breast cancer; advanced hormone receptor positive (HR+), HER2-negative in men and postmenopausal women - second-line with fulvestrant okt.18 Omadacycline tosylate Treatment of community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure okt.18 Siponimod Treatmentinfections (ABSSSI) of secondary in adults progressive multiple sclerosis (SPMS) okt.18 autologous cd34+ cell enriched Treatment of transfusion-dependent β- population that contains thalassaemia (TDT) hematopoietic stem cells transduced with lentiglobin bb305 lentiviral vector encoding the beta-a-t87q-globin gene x okt.18 Fostamatinib Indicated for the treatment of thrombocytopenia okt.18 Dolutegravir / lamivudine Treatment of Human Immunodeficiency Virus type 1 (HIV-1) okt.18 Adeno-associated viral vector Treatment of paediatric patients serotype 9 containing the diagnosed with spinal muscular atrophy human SMN gene (AVXS-101) Type 1 okt.18 Treatment of non-neurological manifestations of acid sphingomyelinase Olipudase alfa deficiency okt.18 Treatment of adult and paediatric patients with locally advanced or Larotrectinib metastatic solid tumours x sep.18 Treatment -

F.No: QA/02/Central Inspection Plan/CT/Rdna/2018 Director
F.No: QA/02/Central Inspection Plan/CT/rDNA/2018 Director General of Health Services Central Drugs Standard Control Organisation Office of Drugs Controller General (India) FDA Bhawan, Kotla Road, New Delhi-110002 (QA Division) Dated: Office Memorandum As a process of Clinical trial oversight. CDSCO-HQ has prepared tentative Central Inspection Plan for the Year 2018 for inspections of the clinical trials permitted with respect tor-DNA products in accordance with the elements of SOP QA-INS-004. You are requested to conduct clinical trial inspections as per plan with the team comprising of inspectors trained in GCP. along with subject expert and an officer from CDSCO-HQ. Drugs inspectors from respecti ve states may also join the inspection team. The concerned COSCO Zonal officers are also requested to confirm the status of clinical trial site before planning the inspection at respective site. The clinical trial inspection report shall be submitted after completion of inspection to this office along with your recommendations for further action by this office. Drugs Controller G neral (India) Encl: 1. Central Inspection Plan for cl inical trial inspections for year 2018 for r-DNA products To: All DDC(l)s of North Zone, West Zone, South Zone, East Zone, Ahmedabad Zone, Hyderabad Zone. Varanasi Sub Zone, Jarnmu Sub Zone, Bangalore Sub zone and Baddi Sub Zone. Copy to: Guard fi le (QA Division & Biological division) PS to DCGI Central Drugs Standard Control Organization Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India FDA Bhavan, ITO, Kotla Road, New Delhi -110002 List of Clinical Trials (rDNA Products) to be inspected in the year 2018 Name of Investigational Sr. -

PRAC Draft Agenda of Meeting 4 -7 March 2013
4 March 2013 EMA/PRAC/141813/2013 Pharmacovigilance Risk Assessment Committee (PRAC) Pharmacovigilance Risk Assessment Committee (PRAC) Agenda of the meeting on 4-7 March 2013 Explanatory notes The Notes give a brief explanation of relevant agenda items and should be read in conjunction with the agenda. EU Referral procedures for safety reasons: Urgent EU procedures and Other EU referral procedures (Items 2 and 3 of the PRAC agenda) A referral is a procedure used to resolve issues such as concerns over the safety or benefit-risk balance of a medicine or a class of medicines. In a referral, the EMA is requested to conduct a scientific assessment of a particular medicine or class of medicines on behalf of the European Union (EU). For further detailed information on safety related referrals please see: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000150.jsp&mid =WC0b01ac05800240d0 Signals assessment and prioritisation (Item 4 of the PRAC agenda) A safety signal is information on a new or incompletely documented adverse event that is potentially caused by a medicine and that warrants further investigation. Signals are generated from several sources such as spontaneous reports, clinical studies and the scientific literature. The evaluation of safety signals is a routine part of pharmacovigilance and is essential to ensuring that regulatory authorities have a comprehensive knowledge of a medicine’s benefits and risks. The presence of a safety signal does not mean that a medicine has caused the reported adverse event. The adverse event could be a symptom of another illness or caused by another medicine taken by the patient. -

Justification
Justification to the Resolution of the Federal Joint Committee (G-BA) on an Amendment of the Pharmaceuticals Directive (AM ‑ RL): Annex XII – Resolutions on the Benefit Assessment of Medicinal Products with New Active Ingredients in Accordance with Section 35a SGB V Damoctocog alfa pegol From 20 June 2019 Contents 1. Legal basis ................................................................................................................ 2 2. Key points of the resolution ..................................................................................... 2 2.1 Additional benefit of the medicinal product in relation to the appropriate comparator therapy ..................................................................................................... 3 2.1.1 Approved therapeutic indication of damoctocog alfa pegol (Jivi®) in accordance with the summary of product characteristics ............................................ 3 2.1.2 Appropriate comparator therapy ................................................................... 3 2.1.3 Extent and probability of the additional benefit .............................................. 5 2.1.4 Summary of the assessment ........................................................................ 6 2.2 Number of patients or demarcation of patient groups eligible for treatment ...... 6 2.3 Requirements for a quality-assured application ................................................ 7 2.4 Treatment costs .............................................................................................. -
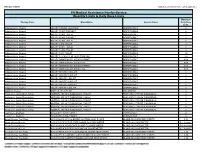
Quantity Limits/Daily Dose Limits
Effective 07/20/21 Alphabetical by Brand Name (when applicable) PA Medical Assistance Fee-for-Service Quantity Limits & Daily Dose Limits Maximum Therapy Class Brand Name Generic Name Daily Dose Limit Antipsychotics, Atypical ABILIFY 1 MG/ML SOLUTION ARIPIPRAZOLE 25 Antipsychotics, Atypical ABILIFY 10 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 10 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 15 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 15 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 2 MG TABLET ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 20 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 30 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 5 MG TABLET ARIPIPRAZOLE 1.5 Antipsychotics, Atypical ABILIFY 9.75 MG/1.3 ML INJECTION VIAL ARIPIPRAZOLE 3.9 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MYCITE 10 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 15 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 2 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 20 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 30 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 5 MG KIT ARIPIPRAZOLE 1 Antivirals, Herpes ABREVA 10% -

Cover Page for Protocol
Cover Page for Protocol Sponsor name: Novo Nordisk A/S NCT number NCT03588741 Sponsor trial ID: NN7170-4345 Official title of study: Evaluation of safety following Immune Tolerance Induction treatment with turoctocog alfa in patients with haemophilia A following inhibitor development in NN7170-4213 trial. Document date: 20-December-2018 Protocol Date: 20 December 2018 Novo Nordisk Trial ID: NN7170-4345 Version: 2.0 CONFIDENTIAL UTN: U1111-1187-7323 Status: Final EudraCT no.: 2016-003821-40 Page: 1 of 74 Protocol Trial ID: NN7170-4345 Evaluation of safety following Immune Tolerance Induction treatment with turoctocog alfa in patients with haemophilia A following inhibitor development in NN7170-4213 trial Final Protocol version 1.0 (18 November 2016), Final Global Protocol Amendment no 1 (19 Dec 2018) Redacted protocol Includes redaction of personal identifiable information only. Trial phase: 3b Protocol originator . This confidential document is the property of Novo Nordisk. No unpublished information contained herein may be disclosed without prior written approval from Novo Nordisk. Access to this document must be restricted to relevant parties.This confidential document is the property of Novo Nordisk. No unpublished information contained herein may be disclosed without prior written approval from Novo Nordisk. Access to this document must be restricted to relevant parties. Protocol Date: 20 December 2018 Novo Nordisk Trial ID: NN7170-4345 Version: 2.0 CONFIDENTIAL UTN: U1111-1187-7323 Status: Final EudraCT no.: 2016-003821-40 Page: -

Mississippi Division of Medicaid Universal Preferred Drug List
MISSISSIPPI DIVISION OF MEDICAID EFFECTIVE 01/01/2021 UNIVERSAL PREFERRED DRUG LIST Version 2021.13a Updated: 8-30-2021 (For All Medicaid, MSCAN and CHIP Beneficiaries) Conduent’s SmartPA Pharmacy Application (SmartPA) is a proprietary electronic prior authorization system used for Medicaid fee for service claims. MSCAN plans may/may not -have electronic PA functionality. However, they must adhere to Medicaid’s PA criteria. THERAPEUTIC PREFERRED AGENTS NON-PREFERRED AGENTS PA CRITERIA DRUG CLASS ACNE AGENTS ANTI-INFECTIVE clindamycin gel (generic Cleocin-T) ACZONE (dapsone) Maximum Age Limit clindamycin lotion AKNE-MYCIN (erythromycin) • 21 years – all agents except clindamycin solution azelaic acid isotretinoins AMZEEQ FOAM (minocycline) AZELEX (azelaic acid) CLEOCIN-T (clindamycin) CLINDAMYCIN PAC (clindamycin) CLINDAGEL (clindamycin) clindamycin foam clindamycin gel daily (generic Clindagel) dapsone ERY (erythromycin) ERYGEL (erythromycin) erythromycin gel, swabs, solution EVOCLIN (clindamycin) KLARON (sulfacetamide) sulfacetamide RETINOIDS RETIN-A (tretinoin) adapalene tretinoin cream AKLIEF (trifarotene) ALTRENO (tretinoin) ARAZLO (tazarotene) ATRALIN (tretinoin) AVITA (tretinoin) DIFFERIN (adapalene) FABIOR (tazarotene) PLIXDA (adapalene) 1 Drug coverage subject to the rules and regulations set forth in Sec. 1927 of Social Security Act.This is not an all-inclusive list of available covered drugs and includes only managed categories. Unless otherwise stated, the listing of a particular brand or generic name includes all dosage forms of that drug. NR indicates a new drug that has not yet been reviewed by the P&T Committee. PREFERRED BRANDS will not count toward the two brand monthly Rx limit. Drugs highlighted in yellow denote a change in PDL status. An * denotes existing users will be grandfathered; grandfathering is defined as approving a Non-Preferred agent for an existing user; all other changes will not qualify for grandfathering. -

Third Quarter 2017 Update
JULY 2017 THIRD QUARTER 2017 UPDATE CHANGES TO THE HIGHMARK DRUG FORMULARIES Following is the Third Quarter 2017 update to the Highmark Drug Formularies and pharmaceutical management procedures. The formularies and pharmaceutical management procedures are updated on a quarterly basis, and the following changes reflect the decisions made in May 2017 by our Pharmacy and Therapeutics Committee. These updates are effective on the dates noted throughout this document. Please reference the guide below to navigate this communication: Section I. Highmark Commercial and Healthcare Reform Formularies A. Changes to the Highmark Comprehensive Formulary and the Highmark Comprehensive Healthcare Reform Formulary B. Changes to the Highmark Progressive Formulary and the Highmark Progressive Healthcare Reform Formulary C. Changes to the Highmark Healthcare Reform Essential Formulary D. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Quantity Level Limit (QLL) Programs Section II. Highmark Medicare Part D Formularies A. Changes to the Highmark Medicare Part D 5-Tier Incentive Formulary B. Changes to the Highmark Medicare Part D 5-Tier Closed Formulary C. Additions to the Specialty Tier D. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Quantity Level Limit (QLL) Program As an added convenience, you can also search our drug formularies and view utilization management policies on the Provider Resource Center (accessible via NaviNet® or our website). Click the Pharmacy/Formulary Information link from the menu on the left Highmark Blue Shield is an independent licensee of the Blue Cross and Blue Shield Association. -

Recent Advances in Developing Specific Therapies for Haemophilia
Recent advances in developing specific therapies for haemophilia Ling G1, Nathwani AC1, Tuddenham EGD1 1Katherine Dormandy Haemophilia and Thrombosis Centre, Royal Free London NHS Foundation Trust, London, UK Summary Therapy of haemophilia has undergone very rapid evolution in the past 10 years. The major limitation of current replacement therapy is the short half-life of factors VIII and IX. These half-lives have been extended by addition of various moieties, allowing less frequent infusion regimens. Entirely novel approaches have also entered the clinic including a bispecific antibody that mimics factor VIII and strategies that rebalance the haemostatic mechanism by reducing antithrombin through inhibition of synthesis. These two treatments are available by subcutaneous injection at infrequent intervals and both can be used in patients with neutralising antibodies (inhibitors). Finally, a cure may be on the horizon with preliminary evidence of success for gene therapy in haemophilia B and A. Key Words: - haemophilia, bleeding disorders, therapy, Factor VIII, Factor IX, gene therapy Short title: Advances in haemophilia therapies Introduction Haemophilia A and B are X-linked, monogenic bleeding disorders due to factor VIII (FVIII) deficiency and factor IX (FIX) deficiency respectively. Approximately 1/5000 males are affected with haemophilia A, with 1/30000 males in haemophilia B (Mannucci & Tuddenham 2001; Bolton-Maggs & Pasi 2003). In its severe form, spontaneous bleeding occurs from a young age, typically in the form of haemarthroses and skeletal muscle haematomas in the absence of antecedent trauma, resulting in chronic arthropathy with significant deformities in the long term if untreated. In the pre-clotting factor concentrate era, death in both conditions was primarily due to intracranial haemorrhage or other life threatening bleeds and patients rarely survived beyond 10 years. -

Xermelo (Telotristat Ethyl)
Prior Authorization Criteria Xermelo (telotristat ethyl) Policy Number: C16325-A CRITERIA EFFECTIVE DATES: ORIGINAL EFFECTIVE DATE LAST REVIEWED DATE NEXT REVIEW DUE BY OR BEFORE 7/2019 12/2/2020 1/26/2022 HCPCS CODING TYPE OF CRITERIA LAST P&T APPROVAL/VERSION J8499 (NOC) RxPA Q1 2021 20210127C16325-A PRODUCTS AFFECTED: Xermelo (telotristat ethyl) DRUG CLASS: Tryptophan hydroxylase inhibitor ROUTE OF ADMINISTRATION: oral PLACE OF SERVICE: Retail Pharmacy AVAILABLE DOSAGE FORMS: 250mg tablets FDA-APPROVED USES: for the treatment of carcinoid syndrome diarrhea in combination with somatostatin analog (SSA) therapy in adults inadequately controlled by SSA therapy COMPENDIAL APPROVED OFF-LABELED USES: None COVERAGE CRITERIA: INITIAL AUTHORIZATION DIAGNOSIS: Carcinoid syndrome diarrhea REQUIRED MEDICAL INFORMATION: A. CARCINOID SYNDROME DIARRHEA: 1. Documentation of member has a carcinoid/neuroendocrine tumor and has a diagnosis of carcinoid syndrome AND 2. Member has been receiving therapy with the FDA-approved maximum (or highest tolerated) dose of a somatostatin analog therapy (SSA) (i.e., octreotide solution/depot or lanreotide depot) for at least 3 months AND 3. Member will continue to receive this SSA therapy in combination with Xermelo; AND 4. Member has had an inadequate response to antidiarrheals (e.g., loperamide); Molina Healthcare, Inc. confidential and proprietary © 2021 This document contains confidential and proprietary information of Molina Healthcare and cannot be reproduced, distributed, or printed without written permission