Evaluation of Special Management Measures for Midcontinent Lesser Snow Geese and Ross’S Geese Report of the Arctic Goose Habitat Working Group
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
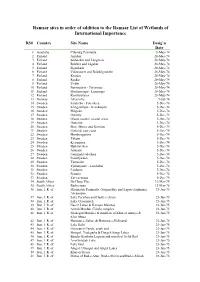
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Proceedings Template
Canadian Science Advisory Secretariat (CSAS) Research Document 2020/032 Central and Arctic Region Ecological and Biophysical Overview of the Southampton Island Ecologically and Biologically Significant Area in support of the identification of an Area of Interest T.N. Loewen1, C.A. Hornby1, M. Johnson2, C. Chambers2, K. Dawson2, D. MacDonell2, W. Bernhardt2, R. Gnanapragasam2, M. Pierrejean4 and E. Choy3 1Freshwater Institute Fisheries and Oceans Canada 501 University Crescent Winnipeg, MB R3T 2N6 2North/South Consulting Ltd. 83 Scurfield Blvd, Winnipeg, MB R3Y 1G4 3McGill University. 845 Sherbrooke Rue, Montreal, QC H3A 0G4 4Laval University Pavillon Alexandre-Vachon 1045, , av. of Medicine Quebec City, QC G1V 0A6 July 2020 Foreword This series documents the scientific basis for the evaluation of aquatic resources and ecosystems in Canada. As such, it addresses the issues of the day in the time frames required and the documents it contains are not intended as definitive statements on the subjects addressed but rather as progress reports on ongoing investigations. Published by: Fisheries and Oceans Canada Canadian Science Advisory Secretariat 200 Kent Street Ottawa ON K1A 0E6 http://www.dfo-mpo.gc.ca/csas-sccs/ [email protected] © Her Majesty the Queen in Right of Canada, 2020 ISSN 1919-5044 Correct citation for this publication: Loewen, T. N., Hornby, C.A., Johnson, M., Chambers, C., Dawson, K., MacDonell, D., Bernhardt, W., Gnanapragasam, R., Pierrejean, M., and Choy, E. 2020. Ecological and Biophysical Overview of the Southampton proposed Area of Interest for the Southampton Island Ecologically and Biologically Significant Area. DFO Can. Sci. Advis. Sec. Res. -

A Historical and Legal Study of Sovereignty in the Canadian North : Terrestrial Sovereignty, 1870–1939
University of Calgary PRISM: University of Calgary's Digital Repository University of Calgary Press University of Calgary Press Open Access Books 2014 A historical and legal study of sovereignty in the Canadian north : terrestrial sovereignty, 1870–1939 Smith, Gordon W. University of Calgary Press "A historical and legal study of sovereignty in the Canadian north : terrestrial sovereignty, 1870–1939", Gordon W. Smith; edited by P. Whitney Lackenbauer. University of Calgary Press, Calgary, Alberta, 2014 http://hdl.handle.net/1880/50251 book http://creativecommons.org/licenses/by-nc-nd/4.0/ Attribution Non-Commercial No Derivatives 4.0 International Downloaded from PRISM: https://prism.ucalgary.ca A HISTORICAL AND LEGAL STUDY OF SOVEREIGNTY IN THE CANADIAN NORTH: TERRESTRIAL SOVEREIGNTY, 1870–1939 By Gordon W. Smith, Edited by P. Whitney Lackenbauer ISBN 978-1-55238-774-0 THIS BOOK IS AN OPEN ACCESS E-BOOK. It is an electronic version of a book that can be purchased in physical form through any bookseller or on-line retailer, or from our distributors. Please support this open access publication by requesting that your university purchase a print copy of this book, or by purchasing a copy yourself. If you have any questions, please contact us at ucpress@ ucalgary.ca Cover Art: The artwork on the cover of this book is not open access and falls under traditional copyright provisions; it cannot be reproduced in any way without written permission of the artists and their agents. The cover can be displayed as a complete cover image for the purposes of publicizing this work, but the artwork cannot be extracted from the context of the cover of this specificwork without breaching the artist’s copyright. -

Arctic Goose Joint Venture STRATEGIC PLAN 2008 – 2012
Arctic Goose Joint Venture STRATEGIC PLAN 2008 – 2012 Arctic Goose Joint Venture STRATEGIC PLAN 2008 – 2012 Cover Photos (clockwise from top left): Doug Steinke, Doug Steinke, John Conkin, Jeff Coats, Tim Moser, Tim Moser, Doug Steinke Arctic Goose Joint Venture Technical Committee. 2008. Arctic Goose Joint Venture Strategic Plan: 2008 - 2012. Unpubl. Rept. [c/o AGJV Coordination Office, CWS, Edmonton, Alberta]. 112pp. Strategic Plan 2008 – 2012 Table of Contents INtroductioN ................................................................................................................ 7 ACCOMPLISHMENts AND FUTURE CHALLENGES .................................................... 9 Past Accomplishments ....................................................................................................... 9 Banding ...................................................................................................................... 9 Surveys ..................................................................................................................... 10 Research ................................................................................................................... 10 Future Challenges ........................................................................................................... 11 INformatioN NEEDS AND Strategies to ADDRESS THEM ............................ 12 Definitions of Information Needs.................................................................................... 12 Strategies for Meeting the Information -

Summary of the Hudson Bay Marine Ecosystem Overview
i SUMMARY OF THE HUDSON BAY MARINE ECOSYSTEM OVERVIEW by D.B. STEWART and W.L. LOCKHART Arctic Biological Consultants Box 68, St. Norbert P.O. Winnipeg, Manitoba CANADA R3V 1L5 for Canada Department of Fisheries and Oceans Central and Arctic Region, Winnipeg, Manitoba R3T 2N6 Draft March 2004 ii Preface: This report was prepared for Canada Department of Fisheries and Oceans, Central And Arctic Region, Winnipeg. MB. Don Cobb and Steve Newton were the Scientific Authorities. Correct citation: Stewart, D.B., and W.L. Lockhart. 2004. Summary of the Hudson Bay Marine Ecosystem Overview. Prepared by Arctic Biological Consultants, Winnipeg, for Canada Department of Fisheries and Oceans, Winnipeg, MB. Draft vi + 66 p. iii TABLE OF CONTENTS 1.0 INTRODUCTION.........................................................................................................................1 2.0 ECOLOGICAL OVERVIEW.........................................................................................................3 2.1 GEOLOGY .....................................................................................................................4 2.2 CLIMATE........................................................................................................................6 2.3 OCEANOGRAPHY .........................................................................................................8 2.4 PLANTS .......................................................................................................................13 2.5 INVERTEBRATES AND UROCHORDATES.................................................................14 -

NTI IIBA for Conservation Areas Cultural Heritage and Interpretative
NTI IIBA for Phase I: Cultural Heritage Resources Conservation Areas Report Cultural Heritage Area: Dewey Soper and Interpretative Migratory Bird Sanctuary Materials Study Prepared for Nunavut Tunngavik Inc. 1 May 2011 This report is part of a set of studies and a database produced for Nunavut Tunngavik Inc. as part of the project: NTI IIBA for Conservation Areas, Cultural Resources Inventory and Interpretative Materials Study Inquiries concerning this project and the report should be addressed to: David Kunuk Director of Implementation Nunavut Tunngavik Inc. 3rd Floor, Igluvut Bldg. P.O. Box 638 Iqaluit, Nunavut X0A 0H0 E: [email protected] T: (867) 975‐4900 Project Manager, Consulting Team: Julie Harris Contentworks Inc. 137 Second Avenue, Suite 1 Ottawa, ON K1S 2H4 Tel: (613) 730‐4059 Email: [email protected] Report Authors: Philip Goldring, Consultant: Historian and Heritage/Place Names Specialist Julie Harris, Contentworks Inc.: Heritage Specialist and Historian Nicole Brandon, Consultant: Archaeologist Note on Place Names: The current official names of places are used here except in direct quotations from historical documents. Names of places that do not have official names will appear as they are found in the source documents. Contents Maps and Photographs ................................................................................................................... 2 Information Tables .......................................................................................................................... 2 Section -

The Structure and Composition of Vegetation in the Lake-Fill Peatlands of Indiana
2001. Proceedings of the Indiana Academy of Science 1 10:51-78 THE STRUCTURE AND COMPOSITION OF VEGETATION IN THE LAKE-FILL PEATLANDS OF INDIANA Anthony L. Swinehart 1 and George R. Parker: Department of Forestry and Natural Resources, Purdue University, West Lafayette, Indiana 47907 Daniel E. Wujek: Department of Biology, Central Michigan University, Mount Pleasant, Michigan 48859 ABSTRACT. The vegetation of 16 lake-fill peatlands in northern Indiana was systematically sampled. Peatland types included fens, tall shrub bogs, leatherleaf bogs and forested peatlands. No significant difference in species richness among the four peatland types was identified from the systematic sampling. Vegetation composition and structure, along with water chemistry variables, was analyzed using multi- variate statistical analysis. Alkalinity and woody plant cover accounted for much of the variability in the herbaceous and ground layers of the peatlands, and a successional gradient separating the peatlands was evident. A multivariate statistical comparison of leatherleaf bogs from Indiana, Michigan, Ohio, New York, New Jersey and New Hampshire was made on the basis of vegetation composition and frequency and five climatic variables. The vascular vegetation communities of Indiana peatlands and other peatlands in the southern Great Lakes region are distinct from those in the northeastern U.S., Ohio and the northern Great Lakes. Some of these distinctions are attributed to climatic factors, while others are related to biogeo- graphic history of the respective regions. Keywords: Peatlands, leatherleaf bogs, fens, ecological succession, phytogeography Within midwestern North America, the such as Chamaedaphne calyculata, Androm- northern counties of Illinois, Indiana and Ohio eda glaucophylla, and Carex oligospermia of- 1 represent the southern extent of peatland com- ten make "southern outlier peatlands ' con- munities containing characteristic plant spe- spicuous to botanists, studies of such cies of northern or boreal affinity. -

Harvest Distribution and Derivation of Atlantic Flyway Canada Geese
Articles Harvest Distribution and Derivation of Atlantic Flyway Canada Geese Jon D. Klimstra,* Paul I. Padding U.S. Fish and Wildlife Service, Division of Migratory Bird Management, 11510 American Holly Drive, Laurel, Maryland 20708 Abstract Harvest management of Canada geese Branta canadensis is complicated by the fact that temperate- and subarctic- breeding geese occur in many of the same areas during fall and winter hunting seasons. These populations cannot readily be distinguished, thereby complicating efforts to estimate population-specific harvest and evaluate harvest strategies. In the Atlantic Flyway, annual banding and population monitoring programs are in place for subarctic- breeding (North Atlantic Population, Southern James Bay Population, and Atlantic Population) and temperate- breeding (Atlantic Flyway Resident Population [AFRP]) Canada geese. We used a combination of direct band recoveries and estimated population sizes to determine the distribution and derivation of the harvest of those four populations during the 2004–2005 through 2008–2009 hunting seasons. Most AFRP geese were harvested during the special September season (42%) and regular season (54%) and were primarily taken in the state or province in which they were banded. Nearly all of the special season harvest was AFRP birds: 98% during September seasons and 89% during late seasons. The regular season harvest in Atlantic Flyway states was also primarily AFRP geese (62%), followed in importance by the Atlantic Population (33%). In contrast, harvest in eastern Canada consisted mainly of subarctic geese (42% Atlantic Population, 17% North Atlantic Population, and 6% Southern James Bay Population), with temperate- breeding geese making up the rest. Spring and summer harvest was difficult to characterize because band reporting rates for subsistence hunters are poorly understood; consequently, we were unable to determine the magnitude of subsistence harvest definitively. -

Canada � Underwater World 2
A fici/ c ARCTIC CHARR Canada Underwater World 2 first cousin to the great fighting trout The most famous of the charrs, of F and the magnificent salmon, the course, is the eastern brook trout — arctic charr is a fish of distinguished Salvelinus fontinalis. This is probably ARCTIC family. In fact, to the uninitiated the dif- one of the best known fishes on the con- ferences between it and the other salmo- tinent since in the days before pollution CHARR nids are so slight as to be negligible — they swam in virtually every river and a scattering of light-coloured dots, a stream to the east of Saskatchewan. Not novel arrangement of teeth and a slight so widely known is the Dolly Varden variation in the bone structure of the malma — a crimson-spotted fish that—S. mouth. Yet to the knowledgeable, these was named in nineteenth century Cali- are the unmistakeable signs that say fornia after the brightly dressed heroine charr. of a Dickens novel. The Dolly Varden During much of the venerable history is found in rivers and streams all around of angling, the charr was considered the Pacific Rim, from California to little more than a poor relation to the Japan. Then there is the lake trout trout. Indeed, a noted American scien- namaycush — a giant which is sought—S. tist wrote in 1887 that "the English for trophies throughout the northern maintain, generally with a note of half of North America. And finally, in implied disparagement, that our eastern the cold, clear waters of the northern brook is not a trout at all, but a charr." hemisphere is the arctic charr The English were right in their facts, alpinus. -
![QAQSAUQTUUQ MIGRATORY BIRD SANCTUARY MANAGEMENT PLAN [January 2020] Acknowledgements](https://docslib.b-cdn.net/cover/4894/qaqsauqtuuq-migratory-bird-sanctuary-management-plan-january-2020-acknowledgements-1324894.webp)
QAQSAUQTUUQ MIGRATORY BIRD SANCTUARY MANAGEMENT PLAN [January 2020] Acknowledgements
QAQSAUQTUUQ MIGRATORY BIRD SANCTUARY MANAGEMENT PLAN [January 2020] Acknowledgements: Current and former members of the Irniurviit Area Co-management Committee (Irniurviit ACMC) developed the following management plan. Members are: Noah Kadlak, Kevin Angootealuk, Jean-Francois Dufour, Willie Adams, Annie Ningeongan, Louisa Kudluk, Armand Angootealuk, Darryl Nakoolak, Willie Eetuk, and Randy Kataluk. Chris Grosset (Aarluk Consulting Ltd.) and Kim Klaczek prepared the outline, document inventory and early drafts. Susan M. Stephenson (CWS) and Kevin J. McCormick (CWS) drafted an earlier version of the management plan in 1986. Ron Ningeongan (Kivalliq Inuit Association, Community Liaison Officer) and Cindy Ningeongan (Interpreter) contributed to the success of the establishment and operation of the Irniurviit ACMC. Suzie Napayok (Tusaajiit Translations) translated into Inuktitut all ACMC documents leading to and including this management plan. Jim Leafloor (CWS), Paul Smith (ECCC), and Grant Gilchrist (ECCC) contributed information and expert review throughout the management planning process. The Irniurviit ACMC and CWS also wish to thank the community of Coral Harbour and all the organizations and people who reviewed this document at any part of the management planning process. Copies of this Plan are available at the following addresses: Environment and Climate Change Canada Public inquiries centre Fontaine Building 12th floor 200 Sacré-Coeur Blvd Gatineau, QC. K1A 0H3 Telephone: 819-938-3860 Toll-free: 1-800-668-6767 (in Canada only) Email: [email protected] Environment and Climate Change Canada – Canadian Wildlife Service Northern Region 3rd Floor, 933 Mivvik Street PO Box 1870 Iqaluit, NU. X0A 0H0 Telephone: 867-975-4642 Environment and Climate Change Canada Protected Areas website: https://www.canada.ca/en/environment-climate-change/services/national-wildlife-areas.html ISBN: [to be provided for final version only] Cat. -

An Overview of the Hudson Bay Marine Ecosystem
10–1 10.0 BIRDS Chapter Contents 10.1 F. GAVIIDAE: Loons .............................................................................................................................................10–2 10.2 F. PODICIPEDIDAE: Grebes ................................................................................................................................10–3 10.3 F. PROCELLARIIDAE: Fulmars...........................................................................................................................10–3 10.4 F. HYDROBATIDAE: Storm-petrels......................................................................................................................10–3 10.5 F. PELECANIDAE: Pelicans .................................................................................................................................10–3 10.6 F. SULIDAE: Gannets ...........................................................................................................................................10–4 10.7 F. PHALACROCORACIDAE: Cormorants............................................................................................................10–4 10.8 F. ARDEIDAE: Herons and Bitterns......................................................................................................................10–4 10.9 F. ANATIDAE: Geese, Swans, and Ducks ...........................................................................................................10–4 10.9.1 Geese............................................................................................................................................................10–5 -

Canadian Data Report of Fisheries and Aquatic Sciences 2262
Scientific Excellence • Resource Protection & Conservation • Benefits for Canadians Excellence scientifique • Protection et conservation des ressources • Bénéfices aux Canadiens DFO Lib ary MPO B bhotheque Ill 11 11 11 12022686 11 A Review of the Status and Harvests of Fish, Invertebrate, and Marine Mammal Stocks in the Nunavut Settlement Area D.B. Stewart Central and Arctic Region Department of Fisheries and Oceans Winnipeg, Manitoba R3T 2N6 1994 Canadian Manuscript Report of Fisheries and Aquatic Sciences 2262 . 51( P_ .3 AS-5 -- I__2,7 Fisheries Pêches 1+1 1+1and Oceans et Océans CanaclUi ILIIM Canadian Manuscript Report of Fisheries and Aquatic Sciences Manuscript reports contain scientific and technical information that contributes to existing knowledge but which deals with national or regional problems. Distribu- tion is restricted to institutions or individuals located in particular regions of Canada. However, no restriction is placed on subject matter, and the series reflects the broad interests and policies of the Department of Fisheries and Oceans, namely, fisheries and aquatic sciences. Manuscript reports may be cited as full-publications. The correct citation appears above the abstract of each report. Each report is abstracted in Aquatic Sciences and Fisheries Abstracts and,indexed in the Department's annual index to scientific and technical publications. Numbers 1-900 in this series were issued as Manuscript Reports (Biological Series) of the Biological Board of Canada, and subsequent to 1937 when the name of the Board was changed by Act of Parliament, as Manuscript Reports (Biological Series) of the Fisheries Research Board of Canada. Numbers 901-1425 were issued as Manuscript Reports of the Fisheries Research Board of Canada.