Stochastic Dynamics of Cyanobacteria in Long‐Term High‐Frequency
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
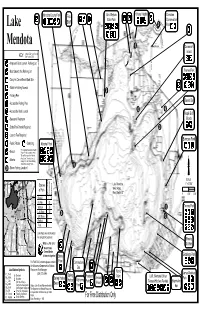
Lake Mendota
wendot2gounty2rk pox qovF2xelson hr2reights gherokee tte2rk gonservtion ry fluff r S yx vke i2 rk vx f 5 xs e xq2yyh2 e 2 i 5 5 r g x e w wendot w y T 5 riivi2hF g qovernor9s sslnd gonttD2pility2nd2eess 5 uiX ht2evised2PHHQ F h 2 E2smproved2fot2vunhD2rking2vot v y y r g E2fot2vunhD2xo2rking2vot rner2rk 5 E2grryEsn2gnoeGmll2fot2ite 5 E2lkEsn2pishing2eess Q E2pishing2ier 5 hunn9s2fr E2eessile2pishing2ier R 5 U E2eessile2fot2vunh wple2fluff E2esonl2estroom 5 5 feh 5 E2tte2rk2ermit2equired V E2vunh2pee2equired furrows2rk E2uli2hone E2gmping wrshll2rk 5 B2por22detiled2desription2of2eh E2feh 229hore2pishing2votion9Y2refer2to 224hore2pishing2on2the2wdison 5 x 22ere2vkesF42evilleD2free2of E2wrin 22hrge2from2the2hr2pishing 5 22glu282hne2gounty2rksF 5 P R E2hore2pishing2votionB 5 i i rieex gevi fexgr 2 vke2wendot I42a2QDSHH9 peies i 5 u wendot e of2pish v WVRP2eres wxF2hepth2VP9 H9 IDPSH9 QDSHH9 eundnt gommon resent wuskie xF2ike 5 lleye 5 IH enney2rk wonon 5 vFwF2fss I 5 5 ingr FwF2fss W 5 nfish igle rout 5 5 reights syv 5 ues e 5 g vke2wps2re2not2intended 5 for2nvigtion2purposesF 5 5 frerly 5 ht2is22pish2gric tF2frs uegons ood2llets 5 gement2floks 5 5 5 @ll2nded2togetherA toney qiddings2rk oint por2pish2gri2votions2plese2ontt gommodore 5 the2isonsin2heprtment2of2xturl fr vke2fottom2ymolsX esoures2pish2wngerX ini 2222222222222222@THVA2PUQESWRT tmes2wdison F et frF fedrok pring prutshi oint FF2wemoril2 nion wkF wuk fF foulders pring2rror rror wuenn eond rk gF gly tumps282ngs feh oint oint erreGroofers2entls idgewter wF wrl hngerous2xvigtion -

Phytoplankton and Primary Production
Phytoplankton and Primary Production Phagotrophic dinoflagellates Cryptomonads & photosynthetic Chain Forming diatoms Diatoms Green algae Blue Green algae (Cyanobacteria) Anabaena Productivity: rate at which organic matter is produced Microcystis Production: Total organic matter produced Relationship Between Primary Productivity Lakes are Categorized According to their Level of and Phytoplankton Biomass (Production) Productivity or “Trophic State” More Productive For conditions in which nutrients are limited Less Productive Biomass Productivity g Carbon . m-3. day-1 (Production) Oligotrophic Mesotrophic Eutrophic g Carbon . m-3 Greater Water Clarity : 10 m Less Water Clarity: 2.5 m Less Algae : 1.7 µg/L Chl a More Algae : > 14 µg/L Chl a Lower Phosphorus : < 8 µg/L More Phosphorus: 16 µg/L time PHOSPHORUS AND CHLOROPHYLL CONCENTRATIONS AND SECCHI DISK DEPTHS CHARACTERISTIC OF THE TROPHIC CLASSIFICATION OF LAKES Phosphorus Concentrations Vary Across the Western Finger Lakes MEASURED PARAMETER Oligotrophic Mesotrophic Eutrophic Total Phosphorus (µg/L) AVG 8 26.7 84.4 Range 3.0- 17.7 10.7- 95.6 16-386 Chlorophyll a (µg/L) AVG 1.7 4.7 14.3 Range 0.3 - 4.5 3 – 11 3 – 78 Secchi Disk Depth (m) AVG 9.9 4.2 2.45 Range 5.4 - 28.3 1.5 – 8.1 0.8 – 7.0 Table I – Data from Wetzel, 1983 Source: CSLAP data Chlorophyll Concentrations Are Relatively Low In Conesus Lake Cycle of Phytoplankton Production and Biomass in Temperate Lakes Source: CSLAP data Start of Spring bloom Autumn bloom fall Summer turnover Dry Period & fallout Surface temperature in a -

TIEE Teaching Issues and Experiments in Ecology - Volume 3, April 2005
TIEE Teaching Issues and Experiments in Ecology - Volume 3, April 2005 ISSUES : DATA SET Changes in Lake Ice: Ecosystem Response to Global Change Robert E. Bohanan, Center for Biology Education, University of Wisconsin – Madison, Madison, WI 53706, [email protected] Marianne Krasny, Department of Natural Resources, Cornell University, Ithaca, NY, 14853, [email protected] Adam Welman, Center for the Environment, Cornell University, Ithaca, NY, 14853 THE ECOLOGICAL QUESTION: Is there evidence for global warming in long term data on changes in dates of ice cover in three Wisconsin Lakes? ECOLOGICAL CONTENT: Effects of climate change on ecological systems. WHAT STUDENTS DO: Students plot more than 100 years of data on dates of "ice on" and "ice off" and duration of ice cover for three Wisconsin Lakes. They examine patterns of variation at different time scales to see the importance of long-term data. SKILLS: Interpreting data, making inferences from trends or patterns in data, making spatial and temporal comparisons of ecological systems. ASSESSABLE OUTCOMES: Interpretation of data, analyzing trends and patterns in spatial and temporal data, and constructing explanations about the links between abiotic and biotic factors on ecological systems from large-scale data. SOURCE: North Temperate Lakes LTER archive (http://lterquery.limnology.wisc.edu/abstract_new.jsp?id=PHYS) TIEE, Volume 3 © 2005 - Robert E. Bohanan and the Ecological Society of America. Teaching Issues and Experiments in Ecology (TIEE) is a project of the Education and Human Resources Committee of the Ecological Society of America (http://tiee.ecoed.net). page 2 Robert E. Bohanan, Marianne Krasny, Adam Welman TIEE Volume 3, April 2005 OVERVIEW Note: the overview is written for faculty but can be used as the basis for an introduction to the data set for students. -

Lakes in Winter
NORTH AMERICAN LAKE NONPROFIT ORG. MANAGEMENT SOCIETY US POSTAGE 1315 E. Tenth Street PAID Bloomington, IN 47405-1701 Bloomington, IN Permit No. 171 Lakes in Winter in Lakes L L INE Volume 34, No. 4 • Winter 2014 Winter • 4 No. 34, Volume AKE A publication of the North American Lake Management Society Society Management Lake American North the of publication A AKE INE Contents L L Published quarterly by the North American Lake Management Society (NALMS) as a medium for exchange and communication among all those Volume 34, No. 4 / Winter 2014 interested in lake management. Points of view expressed and products advertised herein do not necessarily reflect the views or policies of NALMS or its Affiliates. Mention of trade names and commercial products shall not constitute 4 From the Editor an endorsement of their use. All rights reserved. Standard postage is paid at Bloomington, IN and From the President additional mailing offices. 5 NALMS Officers 6 NALMS 2014 Symposium Highlights President 11 2014 NALMS Awards Reed Green Immediate Past-President 15 2014 NALMS Photo Contest Winners Terry McNabb President-Elect 16 2014 NALMS Election Results Julie Chambers Secretary Sara Peel Lakes in Winter Treasurer Michael Perry 18 Lake Ice: Winter, Beauty, Value, Changes, and a Threatened NALMS Regional Directors Future Region 1 Wendy Gendron 28 Fish in Winter – Changes in Latitudes, Changes in Attitudes Region 2 Chris Mikolajczyk Region 3 Imad Hannoun Region 4 Jason Yarbrough 32 A Winter’s Tale: Aquatic Plants Under Ice Region 5 Melissa Clark Region 6 Julie Chambers 38 A Winter Wonderland . of Algae Region 7 George Antoniou Region 8 Craig Wolf 44 Water Monitoring Region 9 Todd Tietjen Region 10 Frank Wilhelm 48 Winter Time Fishery at Lake Pyhäjärvi Region 11 Anna DeSellas Region 12 Ron Zurawell At-Large Nicki Bellezza Student At-Large Ted Harris 51 Literature Search LakeLine Staff Editor: William W. -

AMENDMENT Aquatic Plant Management Plan, Lake Mendota, Lower Rock River Basin, Dane County Wisconsin
AMENDMENT Aquatic Plant Management Plan, Lake Mendota, Lower Rock River Basin, Dane County Wisconsin Approved by the Dane County Lakes and Watershed Commission on April 10, 2014 and by the Wisconsin Department of Natural Resources on March 27, 2014 Prepared by Sue Jones, Dane County Office of Lakes and Watersheds, with assistance from Jim Leverance, Darren Marsh, and Pat Sheahan. Mapping by Michelle Richardson, Dane County Land and Water Resources Department, Administration Division. Plant surveys conducted by James Scharl of Stantec Consulting Services Inc. (2011) for the Dane County Office of Lakes and Watersheds. The Wisconsin Department of Natural Resources provided funding to the Office of Lakes and Watersheds to support this plan amendment. Introduction This is an update to the Aquatic Plant Management Plan, Lake Mendota, Lower Rock River Basin, Dane County Wisconsin, published in January 2007 by the Dane County Office of Lakes and Watersheds. The Wisconsin Department of Natural Resources approved the 2007 plan on March 17, 2007 and the Dane County Lakes and Watershed Commission approved the plan on April 12, 2007. Aquatic Plant Management Plans are required under NR 109.04(d), Wisconsin Administrative Code, to guide mechanical harvesting activities and the effective management of aquatic plants in water bodies. This plan is prepared in support of Dane County’s permit for its mechanical aquatic plant harvesting program, operated in accordance with NR 109 Wisconsin Administrative Code. Individuals and groups that propose herbicide treatments of aquatic plants in Dane County waters would need to go through a separate planning and permitting process with the Wisconsin Department of Natural Resources. -

Evaluation of Cyanotoxins in the Farmington Bay, Great Salt Lake, Utah
Evaluation of Cyanotoxins in the Farmington Bay, Great Salt Lake, Utah. Nodularin Dr. Ramesh Goel Assistant Professor Department of Civil and Environmental Engineering University of Utah & Leland Myers District Manager Central Davis Sewer District 2627 W Shepard Lane Kaysville, UT 84039 (801) 451-2190 Project Report March, 2009 ABSTRACT The presence of toxic cyanobacteria in several marine environments world wide is well documented. However, the presence of cyanobacteria in the Farmington Bay of the Great Salt Lake, Utah, is largely unexplored. Recently, a great concern is being raised over severe eutrophication in the Farmington Bay due to the presence and the inflow of nutrients in the bay from various sources. Excess growth of algae, especially during summer months, has been observed in the Farmington Bay. In brackish waters, such as in the Great Salt Lake, the cyanobaceria belonging to the genus Nodularia generally occurs and produces the toxin nodularin. This study quantified nodularin in representative and homogeneous water samples collected from six different locations in the Farmington Bay of the Great Salt Lake at four different occasions during the summer of 2008. A significant portion of the research was also devoted to analytical method development and quality control tests in our laboratory. An ultra performance liquid chromatography-based analytical method was developed for nodularin found in brackish waters. When deionized water was spiked with nodularin to give a final concentration of 1 μg/L, more than 99 % toxins could be recovered from deionized water, thus demonstrating the ability of the newly developed method to detect and quantify low concentrations of nodularin. -

State of the Lakes Report Shows the Impact and Accelerate the Adoption of Effective Solutions
WHAT CLOSED OUR BEACHES? LAKE BY LAKE REPORT A look at beach closure data and A breakdown of the state of each the cause of closures of the Yahara lakes PG. 43 (CENTER)SOTL PG. 45 - 49 Volunteer water quality monitor Greater Madison Lake Guide training| Summer on 2020Lake Mendota39 STATE OF THE LAKES WHAT HAPPENS ON THE LAND, IMPACTS THE LAKES View of Wisconsin State Capitol over Lake Mendota The Yahara River Watershed encompasses lakes Mendota, large number of beach closures. These conditions can be Monona, Wingra, Waubesa, and Kegonsa, and is home to a tied in part to wetter than normal weather. Large runoff fast-growing population and some of the most productive events, especially in the late winter when the ground was farmland in the United States. The intersection of frozen, contributed to the highest annual phosphorus productive farms and growing urban areas presents unique loading since the 1990s. Overall, phosphorus loading was challenges as we work to improve water quality. 2019 also saw 146 days of beach closures, or 39 more closure days than the long-term median (2010-2019). landscape. Many of the decisions we make on the land Cyanobacteria blooms were the primary cause of the 2019 impact the health of our lakes, from how we manage beach closures, and were likely made worse by invasive manure, to how much winter salt we use. zebra mussels. Zebra mussels (see page 26) consume At Clean Lakes Alliance, we focus on curbing the biggest cyanobacteria—which compete for the same nutrients and culprit of our water quality problems—phosphorus sunlight—largely untouched. -

MONITORING BEACH HEALTH Public Health Madison & Dane County Monitors Dane County Area Beaches for E
MONITORING BEACH HEALTH Public Health Madison & Dane County monitors Dane County area beaches for E. coli bacteria and blue-green algae. Our goal is to prevent harmful bacteria and toxins from making people ill while they enjoy our area lakes and beaches. We test for… E. coli bacteria Waterborne pathogens are most commonly bacteria, viruses or Beaches monitored by Public parasites originating from human or animal feces and are associated with illnesses among swimmers. Health Madison & Dane County Because testing for multiple pathogens is time-consuming and Lake Mendota: expensive, scientists and beach managers use indicator James Madison organisms for monitoring. For freshwaters, Escherichia coli Lake Mendota County Park Marshall (E. coli) is the best indicator for the presence of pathogens that Memorial Union can make people sick. Spring Harbor Tenney Cyanobacteria (blue-green algae) Warner Photosynthetic bacteria, called cyanobacteria can form harmful Lake Monona: algal blooms in freshwater that can be toxic to people and BB Clarke animals, causing a variety of symptoms. Bernies Brittingham Favorable conditions, including high nutrient levels, sunshine Esther and other factors can promote development of dense Frost Woods populations of cyanobacteria. Hudson Olbrich Olin How beach monitoring information is Schluter communicated Lake Waubesa: We close beaches when the presence of E. coli and Goodland cyanobacteria pose a risk to health and well-being. Lake Wingra: www.publichealthmdc.com/beaches Vilas Email notifications Stewart Lake: Sign-up on the beaches website page Stewart Lake /publichealthmdc /publichealthmdc Signs at the beach www.publichealthmdc.com/beaches June 2018 Monitoring Beach Health When do we collect samples? E. -

2018 Yahara Chain of Lakes Flooding Technical Work Group Report
2018 Yahara Chain of Lakes Flooding Technical Work Group Report Photo of flooding in Tenney Park with Lake Mendota in the background. Courtesy of Rick Lange (Dane County Sheriff Office) performing drone footage of flooding in August 2018. February 1, 2019 Table of Contents 1.0 Executive Summary ................................................................................................................................ 1 2.0 Introduction ........................................................................................................................................... 2 2.1 The Yahara Lakes and Flooding .......................................................................................................... 4 2.2 2018 Water Levels and Management ................................................................................................ 7 3.0 Technical Approach ................................................................................................................................ 9 3.1 INFOS Framework .............................................................................................................................. 9 3.2 INFOS Model Performance .............................................................................................................. 10 3.2.1 Comparison between Modeled and Observed Lake Levels ...................................................... 10 3.2.2 Comparison between Modeled and Observed River Water Surface Profiles ........................... 11 3.2.3 Comparison between Modeled -

AQUATIC PLANT MANAGEMENT PLAN LAKE MENDOTA Lower
AQUATIC PLANT MANAGEMENT PLAN LAKE MENDOTA Lower Rock River Basin DANE COUNTY, WISCONSIN Madison’s Isthmus and Lake Mendota January 2007 OFFICE OF LAKES AND WATERSHEDS DANE COUNTY LAND AND WATER RESOURCES DEPARTMENT 1 FEN OAK COURT, RM 234 MADISON, WI 53718 Prepared by Underwater Habitat Investigations, LLC (Dave Marshall) and Dane County Land & Water Resources and Public Works Staff (Kevin Connors, Deb Flanders, Teresa Haynes, Sue Jones, Jim Leverance, Darren Marsh, Michelle Richardson, Joe Yaeger) Edited by Sue Jones Cover photo by Richard Lathrop Preparation of this plan was funded in part by a Wisconsin Department of Natural Resources Lake Planning Grant This plan was approved by the Wisconsin Department of Natural Resources on March 17, 2007 and by the Dane County Lakes and Watershed Commission on April 12, 2007 2 Summary An aquatic plant survey was performed during the summer of 2006 on Lake Mendota. A total of 633 sites were sampled across the lake. Results of the point intercept survey indicated that coontail and Eurasian watermilfoil were the most frequently collected plants in 2006, as they were from 1989 – 1991. Coontail was the dominant plant in 2006 in terms of relative frequency, occurrence and density (rake fullness). Native species richness (the number of different species present) was higher in 2006 than in the most recent surveys conducted from 1989 through 1991. Additional species found in 2006 included horned pondweed and muskgrass. Overall densities of aquatic plants did not create significant nuisance problems in 2006. Very low plant densities were found in some areas. In the bay where the Yahara River and Six Mile Creek enter, poor water clarity and strong wind fetch may have contributed to low abundance of plants. -

Lake Access, Mooring and Canoe & Kayak Storage
Canoe & Kayak Storage Lake Access Permit Rules & Tips for Safe Boating A variety of outdoor waterfront park locations The Lake Access Permit is a year-round permit » Life Preserver - A U.S. Coast Guard life are available to store canoes and kayaks. required for the use of all designated launch preserver for each person on board is Contact for available locations and rates. sites in the city of Madison, city of Monona, required on vessels 16 ft. and longer, and Lake Access, and Dane County Parks (see map). Permits are must be readily accessible. Park Location valid for the issue year through March 31 of the » Lights - must be ON sunset to sunrise. following year and must be displayed on the BB Clarke Beach 835 Spaight St. » Battery - must be covered and secured Mooring and inside driver’s side windshield. Brittingham 701 W. Brittingham Pl. against shifting. Cherokee 1000 Burning Wood Way » Mufflers - Motorboats must be equipped Canoe & Kayak Esther Beach 2802 Waunona Way PURCHASE PERMITS ONLINE AT: with mufflers. James Madison 614 E. Gorham St. » Registration - must be current and cityofmadison.com/parks/permits displayed properly. Law 410 S. Blair St. Storage » Symbols - Know and respect waterway Marshall 2101 Allen Blvd. symbols and dive flags. Merrill Springs 5102 Spring Ct. Parking » Wake - Adhere to the 200 ft. no wake zone Olbrich 3527 Atwood Ave. No Grass restriction along the shoreline and 100 ft. Paunack 1500 Waunona Way Parking on the grass is prohibited at ALL of no wake area around police/fire boats Spring Harbor 5218 Lake Mendota Dr. -

Lake Mendota
Lake Mendota elevation: approximately 850 feet above mean sea level (for daily levels, see: www.countyofdane.com/ landconservation/ lakelevelpg.aspx) 9,842 acres 82-foot max. depth 42-foot mean depth 14 Yahara Waterways – Water Trail Guide Lake Mendota Yahara Waterways – Water Trail Guide 15 ake Mendota is the largest of the Yahara Lakes – three times larger than Lake LMonona. Its watershed covers 217 square miles. Mendota is a glacial lake that formed 15,000 years ago. Although much larger during the last glacial period, the lake shrank as ice reformed its basin. The ancient lakebed is evident in sediments bordering the present day lakeshore. At its largest the lake covered University Bay, most of the Isthmus, Tenney Park, Warner Park, Truax Field, Cherokee Marsh and much of Middleton. Lake Mendota was originally mesotrophic (moderately fertile). As the area became more intensively farmed and experienced more urban development, nutrients and sediments washing into the lake turned it eutrophic, or overly nu- trient-rich. Aquatic plants became more abundant and periodic algae blooms occurred more frequently. However, water quality was worse in the past than it is today. While recent efforts have improved the lake’s quality overall, especially in the area of weed growth, phosphorus and sediment remain problems. Wetlands once covered extensive areas around the lake. They have been filled in, drained, or lost when the lake level was raised with the construction of the Tenney Park dam. Wetlands act as detention ponds, storing polluted runoff and filtering out nutrients before releasing the water back into lakes and streams.