Thermal Stabilization of Recycled PET Through Chain Extension and Blending with PBT
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tape-Unyte High Density Ptfe Tape
TAPE-UNYTE® HIGH DENSITY PTFE TAPE T-TAPE-SPEC PRODUCT DESCRIPTION COLOR/CONSISTENCY TENSILE STRENGTH - LONGITUDINAL 3000 PSI max - ASTM D882 (mod) PRODUCT The tape is white in color. It shall be free of visible voids, cracks, folds, ELONGATION TAPE-UNYTE® High Density PTFE Tape contamination and has consistent physical properties. TAPE-UNYTE® has an 50% min - ASTM D882 (mod) TYPE indefinite shelf life. THICKNESS ® TAPE-UNYTE is a heavy duty, all TEMPERATURE RANGE USE purpose, non-seizing, PTFE thread sealing .0040 ± .0005 Inches - ASTM D374-42 -A compound in tape form that produces a Gases: PACKAGING leakproof seal on all types of metal and -450EF (-268EC) to 500EF (260EC) plastic threaded connections. TAPE- UNYTE® is a high density, 4 MIL PTFE Liquids: U.S. Measure: (polytetrafluoroethylene) Tape supplied on -450EF (-268EC) to 500EF (260EC) finished spools. Stock Code Size PRESSURE RANGE USE F520 ¼" x 520" (.63 cm x 13.2 m) RECOMMENDED USES T260 ½" x 260" (1.27 cm x 6.6 m) Gases: T520 ½” x 520" (1.27 cm x 13.2 m) TAPE-UNYTE® will not transfer any taste up to 10,000 PSI (1450 kPa) T1296 ½” x 1296" (1.27 cm x 32.9 m) or odor to the system being sealed. Liquids: W260 ¾" x 260" (1.9 cm x 6.6 m) Excellent for food and water systems. up to 3,000 PSI (435 kPa) W520 ¾" x 520" (1.9 cm x 13.2 m) TAPE-UNYTE® will never harden, and is an X260 1" x 260" (1.9 cm x 6.6 m) anti-galling tape making it possible to The system may be pressurized disassemble pipes and bolts easily after immediately after assembly. -

Transparent PC/PMMA Blends Via Reactive Compatibilization in a Twin-Screw Extruder
polymers Article Transparent PC/PMMA Blends Via Reactive Compatibilization in a Twin-Screw Extruder Tobias Bubmann 1, Andreas Seidel 2 and Volker Altstädt 1,3,* 1 Department of Polymer Engineering, University of Bayreuth, Universitätsstraße 30, Bayreuth 95447, Germany; [email protected] 2 Covestro Deutschland AG, Business Unit Polycarbonates, Research & Development, Development Blends, Leverkusen 51365, Germany; [email protected] 3 Bavarian Polymer Institute and Bayreuth Institute of Macromolecular Research; University of Bayreuth, Universitätsstraße 30, Bayreuth 95447, Germany * Correspondence: [email protected]; Tel.: +49-(0)-921-55-7471 Received: 6 November 2019; Accepted: 7 December 2019; Published: 12 December 2019 Abstract: The effect of different catalysts on reactive compatibilization of 50/50 polycarbonate (PC)/polymethylmethacrylate (PMMA) blends achieved via transesterification that occurs during compounding in a twin-screw extruder was investigated on a phenomenological (optical and mechanical properties), mesoscopic (phase morphology), and molecular level (PC-graft(g)-PMMA-copolymer formation and polymer molecular weight degradation). Formation of PC-(g)-PMMA-copolymer by transesterification resulting in transparent mono-phase PC/PMMA blends with obviously improved compatibility of the two polymer constituents requires use of a suitable catalyst. As a side-effect, PC-(g)-PMMA-copolymer formation by transesterification is always accompanied by a significant simultaneous decomposition of the molecular weight (Mw) of the PC. For the first time, a colorless, transparent (mono-phase) PC/PMMA 50/50 blend was achieved by a twin-screw extrusion process that can be easily transferred into industrial scale. To achieve this milestone, 0.05 wt% of a weakly acidic phosphonium salt catalyst had to be applied. -

Table of Contents
Table of Contents Page Page Flare & Compression - Nut Assemblies Coupling for use w/ Copper Flare Outlets Connections .................................................................. 3 74755 Series ........................................................ 16-17 74750 Series ..............................................................17 74750S Series ............................................................17 Straight Couplings 74776 Series ..............................................................17 74753 Series ................................................................ 4 74776S Series ............................................................17 74754 Series ................................................................ 5 74758 Series .............................................................6-9 74755 Series ..............................................................15 Di-Electric Unions 74756 Series ..............................................................21 74755DB Series ..........................................................16 74758DB Series ............................................................ 9 3 Part Union w/ Internal Pipe Stop 74759 Series ..............................................................10 Y-Branches 708Y Series ........................................................... 18-19 Tees 74760 Series ........................................................ 10-11 74762, 74763, & 74764 Series..................................12 U-Branches 708U Series ......................................................... -

Tribology of Polymer Blends PBT + PTFE
materials Article Tribology of Polymer Blends PBT + PTFE Constantin Georgescu 1,* , Lorena Deleanu 1,*, Larisa Chiper Titire 1 and Alina Cantaragiu Ceoromila 2 1 Department of Mechanical Engineering, Faculty of Engineering, “Dunarea de Jos” University of Galati, 800008 Galati, Romania; [email protected] 2 Department of Applied Sciences, Cross-Border Faculty, “Dunarea de Jos” University of Galati, 800008 Galati, Romania; [email protected] * Correspondence: [email protected] (C.G.); [email protected] (L.D.); Tel.: +40-743-105-835 (L.D.) Abstract: This paper presents results on tribological characteristics for polymer blends made of polybutylene terephthalate (PBT) and polytetrafluoroethylene (PTFE). This blend is relatively new in research as PBT has restricted processability because of its processing temperature near the degradation one. Tests were done block-on-ring tribotester, in dry regime, the variables being the PTFE concentration (0%, 5%, 10% and 15% wt) and the sliding regime parameters (load: 1, 2.5 and 5 N, the sliding speed: 0.25, 0.5 and 0.75 m/s, and the sliding distance: 2500, 5000 and 7500 m). Results are encouraging as PBT as neat polymer has very good tribological characteristics in terms of friction coefficient and wear rate. SEM investigation reveals a quite uniform dispersion of PTFE drops in the PBT matrix. Either considered a composite or a blend, the mixture PBT + 15% PTFE exhibits a very good tribological behavior, the resulting material gathering both stable and low friction coefficient and a linear wear rate lower than each component when tested under the same conditions. Keywords: polybutylene terephthalate (PBT); polytetrafluoroethylene (PTFE); blend PBT + PTFE; block-on-ring test; linear wear rate; friction coefficient Citation: Georgescu, C.; Deleanu, L.; Chiper Titire, L.; Ceoromila, A.C. -

United States Patent (19) 11 Patent Number: 5,318,854 Hamersma Et Al
US005318854A United States Patent (19) 11 Patent Number: 5,318,854 Hamersma et al. 45) Date of Patent: Jun. 7, 1994 (54) POLYMER MIXTURE BASED ON A 3,644,574 2/1972 Jackson, Jr. et al. ............... 260/873 POLYBUTYLENE TEREPHTHALATE 3,657,389 4/1972 Caldwell ........... ... 525/176 ESTER AND A S-MA COPOLYMER AND 3,728,212 4/1973 Caldwell ... ... 525/176 4,080,354 3/1978 Kramer ..... ... 260/40R FILMS MADE THEREOF 4,126,602 11/1978 Sakee .................... ... 260/40R 75) Inventors: Wilhelmus J. L. A. Hamersma, 4,346,195 8/1982 Hornbaker et al. ................. 52.5/176 Bergen op Zoom, Netherlands; 4,386,186 5/1983 Maresca et al. ... 525/68 4,429,076 l/1984 Saito et al. ... 52.5/57 Roger W. Avakian, Brasschaat, 4,493,919 1/1987 Durbinet al. ... ... 524/505 Belgium 4,497,924 2/1985 Robeson et al. .. ... 524/151 73) Assignee: General Electric Co., Pittsfield, Mass. 4,526,923 7/1985 Hornbaker et al. ... 525/502 4,582,876 4/1986 Weemes ............ ... 525/64 (21) Appl. No.: 1,949 4,665,122 5/1987 Robeson et al. .. ... 524/504 (22 Filed: Jan. 4, 1993 4,717,752 1/1988 Yates, III et al. ................... 52.5/175 FOREIGN PATENT DOCUMENTS Related U.S. Application Data 1029145 4/1978 Canada . 63 Continuation of Ser. No. 671,638, Mar. 20, 1991, aban 004:2724 12/1981 European Pat. Off. doned, which is a continuation of Ser. No. 518,251, 308179 3/1989 European Pat. Off. May 7, 1990, abandoned, which is a continuation of 359565 3/1990 European Pat. -
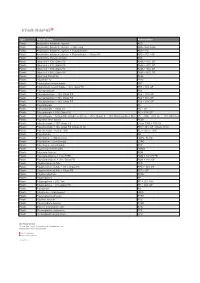
Type Material Name Abbreviation Plastic Acrylonitrile Butadiene
Type Material Name Abbreviation Plastic Acrylonitrile butadiene styrene ABS Plastic Acrylonitrile butadiene styrene - High-Temp ABS - high temp Plastic Acrylonitrile butadiene styrene + Polycarbonate ABS + PC Plastic Acrylonitrile butadiene styrene + Polycarbonate + Glass Fill ABS + PC + GF Plastic Acrylonitrile styrene acrylate ASA Plastic Nylon 6-6 + 10% Glass Fill PA66 + 10% GF Plastic Nylon 6-6 + 20% Glass Fill PA66 + 20% GF Plastic Nylon 6-6 + 30% Glass Fill PA66 + 30% GF Plastic Nylon 6-6 + 50% Glass Fill PA66 + 50% GF Plastic Nylon 6-6 Polyamide PA66 Plastic Polyamide 12 PA12 Plastic Polybutylene terephthalate PBT Plastic Polybutylene terephthalate + 30% Glass Fill PBT+ 30% GF Plastic Polycaprolactam PA6 Plastic Polycaprolactam + 20% Glass Fill PA6 + 20% GF Plastic Polycaprolactam + 30% Glass Fill PA6 + 30% GF Plastic Polycaprolactam + 50% Glass Fill PA6 + 50% GF Plastic Polycarbonate PC Plastic Polycarbonate + Glass Fill PC + GF Plastic Polycarbonate + 10% Glass Fill PC + 10% GF Plastic Polycarbonate + Acrylonitrile butadiene styrene + 20% Glass Fill + 10% Stainless Steel fiber PC + ABS + 20% GF + 10% SS Fiber Plastic Polyether ether ketone PEEK Plastic Polyetherimide + 30% Glass Fill Ultem 1000 + 30% GF Plastic Polyetherimide + 40% Glass Fill (Ultem 2410) PEI + 40% GF (Ultem 2410) Plastic Polyetherimide + Ultem 1000 PEI + Ultem 1000 Plastic Polyethylene PE Plastic Polyethylene - High-Density HDPE, PEHD Plastic Polyethylene - Low-Density LDPE Plastic Polyethylene terephthalate PET Plastic Polymethyl methacrylate PMMA Plastic Polyoxymethylene -

Spherical Polybutylene Terephthalate (PBT)—Polycarbonate (PC) Blend Particles by Mechanical Alloying and Thermal Rounding
polymers Article Spherical Polybutylene Terephthalate (PBT)—Polycarbonate (PC) Blend Particles by Mechanical Alloying and Thermal Rounding Maximilian A. Dechet 1,2,3,†, Juan S. Gómez Bonilla 1,2,3,†, Lydia Lanzl 3,4, Dietmar Drummer 3,4, Andreas Bück 1,2,3, Jochen Schmidt 1,2,3 and Wolfgang Peukert 1,2,3,* 1 Institute of Particle Technology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Cauerstraße 4, D-91058 Erlangen, Germany; [email protected] (M.A.D.); [email protected] (J.S.G.B.); [email protected] (A.B.); [email protected] (J.S.) 2 Interdisciplinary Center for Functional Particle Systems, Friedrich-Alexander-Universität Erlangen-Nürnberg, Haberstraße 9a, D-91058 Erlangen, Germany 3 Collaborative Research Center 814—Additive Manufacturing, Am Weichselgarten 9, D-91058 Erlangen, Germany; [email protected] (L.L.); [email protected] (D.D.) 4 Institute of Polymer Technology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Am Weichselgarten 9, D-91058 Erlangen, Germany * Correspondence: [email protected]; Tel.: +49-9131-85-29400 † The authors contributed equally. Received: 22 November 2018; Accepted: 7 December 2018; Published: 11 December 2018 Abstract: In this study, the feasibility of co-grinding and the subsequent thermal rounding to produce spherical polymer blend particles for selective laser sintering (SLS) is demonstrated for polybutylene terephthalate (PBT) and polycarbonate (PC). The polymers are jointly comminuted in a planetary ball mill, and the obtained product particles are rounded in a heated downer reactor. The size distribution of PBT–PC composite particles is characterized with laser diffraction particle sizing, while the shape and morphology are investigated via scanning electron microscopy (SEM). -

Polymer Properties and Classification
Polymer Characterization LDPE Polyethylene low density HDPE Polyethylene high density ABS Acrylonitrile-butadiene-styrene SAN Styrene-acrylonitrile copolymer EVA Polyethylene co-vinyl acetate PVA Polyvinyl acetate PerkinElmer Solutions for Polymer Characterization Tg(ºC): -130 to 100 Cp (J/g*K): 1,8 to 3,4 Tg(ºC): -130 to 100 Cp (J/g*K): 1,8 to 3,4 Tg(ºC): 110 to 125 CpJ/(g*K): 1,25 to 1,7 Tg(ºC): 95 to 110 CpJ/(g*K): 1,2 Tg(ºC): -45 to 20 CpJ/(g*K): 2,3 Tg(ºC): 25 to 35 CpJ/(g*K): - Tm(ºC): 100 to 120 DHf (J/g): - Tm(ºC): 130 to 140 DHf (J/g): 293 Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm(ºC): 30 to 100 DHf (J/g): 10 to 100 Tm( ºC ): - DHf (J/g): - Td(ºC): 490 to 500 Td(ºC): 490 to 500 Td(ºC): 420 Td(ºC): 420 Td(ºC): 480 Td(ºC): - PP Polypropylene PS PMMA Polymethylmethacrylate PBMA CA Polystyrene Polybuthylmethacrylate Cellulose acetate EP Epoxy resin Molecular Spectroscopy FTIR Differential Scanning Calorimetry Tg(ºC): -20 to -5 CpJ/(g*K): 1,8 Tg(ºC): 90 to 110 Cp (J/g*K): 1,8 to 3,4 Tg(ºC): 85 to 100 CpJ/(g*K): 1,45 to 1,5 Tg(ºC): 15 to 25 CpJ/(g*K): - Tg(ºC): 45 to 60 CpJ/(g*K): - Tg(ºC): 50 to 200 CpJ/(g*K): 1,6 to 2,1 Identify and quantitate organic molecules and compounds, Glass transition & melting temperatures, crystallinity, heat of Understand chemical & physical composition of laminates & fusion, reaction rates, specific heat & heat capacity, curing, Tm(ºC): 165 to 175 DHf (J/g): 207 Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm(ºC): - DHf (J/g): - Tm( ºC): - DHf (J/g): - adhesives , Troubleshoot -

United States Patent (19) 11) E Patent Number: Re
United States Patent (19) 11) E Patent Number: Re. 31,780 Cooper et al. (45) Reissued Date of Patent: Dec. 25, 1984 54 MULTILAYER LIGHT-REFLECTING FILM 56) References Cited (75) Inventors: Scott A. Cooper, Yorktown Heights; U.S. PATENT DOCUMENTS Ramakrishna Shetty, White Plains, 3,479,425 l/1969 Lefevre et al. ..................... 264/171 both of N.Y.; Jules Pinsky, 3,511,903 5/1970 Glass et al. ....... ... 264/73 X Bloomfield, Conn. 3,565,985 2/1971 Schrenk et al. ..................... 264/171 3,759,647 9/1973 Schrenk et al. ... ... 264/7 X 73) Assignee: The Mearl Corporation, Ossining, 3,773,882 1 1/1973 Schrenk .............................. 264/171 N.Y. 3,801,429 4/1974 Schrenk et al. ..................... 428392 (21) Appl. No.: 521,127 4,218,510 8/1980 Willson ........................... 428/483 X 22 Filed: Aug. 8, 1983 FOREIGN PATENT DOCUMENTS 114 1981 2/1969 United Kingdom ................ 428/22 Related U.S. Patent Documents Primary Examiner-Thomas J. Herbert Reissue of: Attorney, Agent, or Firm-Ostrolenk, Faber, Gerb & (64) Patent No.: 4,310,584 Soffen Issued: Jan. 12, 1982 Appl. No.: 107,351 57 ABSTRACT Filed: Dec. 26, 1979 Improvements in multilayer light-reflecting film are 51) Int. Cl. ........................ B32B 7/02; B32B 27/06; effected by the use of thermoplastic polyester as the B32B 27/36 high refractive index component of a system in which (S2) U.S. Cl. .................................... 428/212; 264/171; two or more resinous materials form a plurality of lay 264/173; 350/166; 428/213; 428/480; 428/483 es. 58) Field of Search .............. -

What Is the Difference Between PEX and PB Pipes
Differences Between Crosslinked Polyethylene (PEX) and Polybutylene (PB) Piping Systems for Potable Water Plumbing Applications TN-31 2017 Foreword DIFFERENCES BETWEEN CROSSLINKED POLYETHYLENE (PEX) AND POLYBUTYLENE (PB) PIPING SYSTEMS FOR POTABLE WATER PLUMBING APPLICATIONS This technical note was developed and published with the technical help and financial support of the members of the Plastics Pipe Institute (PPI). These members have shown their commitment to developing and improving quality products by assisting standards development organizations in the development of standards, and also by developing design aids and reports to help engineers, code officials, specifying groups, contractors and users. The purpose of this technical note is to provide information regarding the material and performance differences between PEX and PB piping systems for potable water applications. The PPI has prepared this technical note as a service to the industry. The information in this note is offered in good faith and believed to be accurate at the time of its preparation, but is offered “as is” without any express or implied warranty, including WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE. Additional information may be needed in some areas, especially with regard to unusual or special applications. Consult the manufacturer or material supplier for more detailed information. A list of member manufacturers is available on the PPI website. PPI does not endorse the proprietary products or processes of any manufacturer and assumes no responsibility for compliance with applicable laws and regulations. PPI intends to revise this technical note within 5 years or sooner if required, from the date of its publication, in response to comments and suggestions from users of the document. -

Preparation of Maleic Anhydride Grafted Poly(Trimethylene Terephthalate) (PTT-G-MA) by Reactive Extrusion Processing
Journal of Manufacturing and Materials Processing Article Preparation of Maleic Anhydride Grafted Poly(trimethylene terephthalate) (PTT-g-MA) by Reactive Extrusion Processing Natália F. Braga 1,* , Henrique M. Zaggo 1, Thaís L. A. Montanheiro 2 and Fabio R. Passador 1 1 Laboratório de Tecnologia de Polímeros e Biopolímeros, Universidade Federal de São Paulo - UNIFESP, São José dos Campos, SP 12231-280, Brazil; [email protected] (H.M.Z.); [email protected] (F.R.P.) 2 Laboratório de Plasmas e Processos, Divisão de Ciências Fundamentais, Instituto Tecnológico de Aeronáutica – ITA, São José dos Campos, SP 12228-900, Brazil; [email protected] * Correspondence: [email protected] Received: 5 March 2019; Accepted: 29 April 2019; Published: 4 May 2019 Abstract: Maleic anhydride (MA) grafted with poly(trimethylene terephthalate) (PTT)—abbreviated as PTT-g-MA—can be used as a compatibilizing agent to improve the compatibility and dispersion of nanofillers and a dispersed polymer phase into PTT matrix. This work suggests the preparation of PTT-g-MA using a mixture of PTT, MA, and benzoyl peroxide (BPO) by a reactive extrusion process. PTT-g-MA was characterized to confirm the grafting reaction of maleic anhydride on PTT chains by Fourier transform infrared (FTIR) spectroscopy. Thermal properties (differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA)) and rheological analysis (parallel plates rheology) were used to prove the changes that occurred after the graphitization reaction. The reactive processing route allowed the production of the compatibilizing agent (PTT-g-MA) with good thermal properties and with lower viscosity compared to neat PTT, and this could be an alternative for the compatibilization of polymer blends, as example for PTT/ABS (acrylonitrile butadiene styrene) blends and nanocomposites based on PTT matrix. -

Modified PC/ASA/PMMA Blends with Improved Compatibility, Mechanical Properties and Biocompatible Synergistic Effects with Natural Rubber
Modified PC/ASA/PMMA blends with improved compatibility, mechanical properties and biocompatible synergistic effects with natural rubber Wenfa Dong1, Dingfang Chen1, Ruogu Tang2,3 1. Beishi Petrol Co.LTD. Ningbo, China. 315204 2. Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Science. Ningbo, China. 315204 3. Department of Chemistry, University of Massachusetts, Lowell. Lowell, USA.01854 Abstract In this study, ABS-g-MAH was used as compatibilizer in poly carbonate/acrylonitrile styrene acrylate/poly(methyl methacrylate) blends. The polymer blends were prepared via a two- step method, raw PC, ASA, PMMA resins and ABS-g-MAH additives were mixed and granulated by extrusion molding and then the standard samples were obtained by injection molding. A comprehensive characterizations were performed on the PC/ASA/PMMA blends of their morphologies, mechanical properties and thermal properties. The results showed that the addition of ABS-g-MAH could promote the compatibilities among PC, ASA and PMMA, and ABS- g-MAH would significantly alter the mechanical and thermal properties of blends. In addition, the modified blend was compatible when mixing with natural rubber. The biocompatibility XTT test showed the ABS-g-MAH compatibilized PC derivatives were not cytotoxic. It could be concluded that the appropriate usage of ABS-g-MAH could bring expected benefits on PC/ASA/PMMA blends. Key words: polymer blends, ABS-g-MAH, mechanical strength, stress, segment relaxation, compatibility, cytotoxicity 1. Introduction Poly(carbonate/acrylonitrile styrene acrylate) is a relative new types of polymer blends and it has proved to be a good supplement and alternative to PC/ABS[1-3], this blend combines the advantages of polycarbonate and ASA, however, like many other polymer blends, the compatibility and related mechanical properties are not negligible[4,5].