Efficacy of B12 Fortified Nutrient Bar and Yogurt in Improving Plasma B12 Concentrations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Land Movements in India Farmers Struggle Against Land Grab in PUNE DISTRICT
Land Movements in India an online resource for land rights activists Farmers Struggle against Land Grab in PUNE DISTRICT OCT 27 Posted by jansatyagraha In Pune district, the government has approved 54 SEZs for private sector industries such as Syntel International, Serum Institute, Mahindra Realty, Bharat Forge, City Parks, InfoTech Parks, Raheja Coroporation, Videocon and Xansa India. All SEZs are located around Pune, in areas like Pune Nashik National Highway, Pune-Bangalore National Highway, Pune Hyderabad National Highway and Pune Mumbai Highway. The MIDC has identified 7,500 hectares of agricultural land for procurement in the name of SEZ creation in Pune. Opposition to SEZs has become apparent in many areas, including Karla near Lonavala, Khed- Rajgurunagar, Wagholi at Pune-Aurangabd highway and Karegaon near the Ranjangaon MIDC. It is particularly strong in the Khed taluka district of Pune, where farmers from Gulani, Wafgaon, Wakalwadi, Warude, Gadakwadi, Chaudharwadi, Chinchbaigaon, Jaulake Budruk, Jarewadi, Kanesar, Pur, Gosasi, Nimgaon, Retwadi, Jaulake Khurd, Dhore Bhamburwadi and Pabal face loss of their only source of livelihood from the creation of the Bharat Forge SEZ. These communities, primarily Maratha, OBC and adivasi, are chiefly engaged in agricultural activities. Their major crops are potato, onion, sorghum, jowar, rice, flowers and pulses. Many village youth have also initiated small-scale businesses like poultry, milk collection and pig raring. Although these villages are near the Bhima River basin and surrounded by a small watershed, the government’s lack of investment in infrastructure has left local farmers dependent on unreliable tanker water. Instead of meeting demands for sustainable irrigation schemes to improve the conditions of local farmers, the government seeks to reduce the land of local citizens in order to create an SEZ. -

Pune (Maharashtra) Pin – 412 403
Track ID : MHCOGN26417 Shikshan Prasarak Mandal’s SHRI PADMAMANI JAIN COLLEGE OF ARTS & COMMERCE Pabal Tal-Shirur, Dist- Pune (Maharashtra) Pin – 412 403 Affiliated to Savitribai Phule Pune University, Pune (ID/NO/PU/PN/AC/148/2000) SELF STUDY REPORT 2015 CYCLE 1 Submitted for Accreditation To NATIONAL ASSESSMENT AND ACCREDITATION COUNCIL, BANGALURU Shri Padmamani Jain Arts & Commerce College, Pabal 1 | Page Shri Padmamani Jain Arts & Commerce College, Pabal 2 | Page Shri Padmamani Jain Arts & Commerce College, Pabal 3 | Page CONTENT Sr. No. Details Page No. 1. Preface 5 2. NAAC Steering committee 6 3. Executive Summary & SWOC Analysis 6-13 4. Self Study Report Institutional Data A. Profile of the Institution 14-24 B. Criteria wise analytical report 25-129 1. Criterion I Curricular Aspects 25-38 2. Criterion II Teaching, Learning and Evaluation 39-58 3. Criterion III Research, Consultancy and Extension 59-81 4. Criterion IV Infrastructure and Learning Resources 82-94 5. Criterion V Student Support and Progression 95-106 6. Criterion VI Governance , Leadership and Management 107-120 7. Criterion VII Innovations and Best Practices 121-129 C. Inputs from the Departments Department of Marathi 130-136 Department of English 137-143 Department of Hindi 144-149 Department of Economics 150-159 Department of Political Science 160-167 Department of History 168-175 Department of Geography 176-181 Department of Commerce 182-191 5 IEQA submitted to NAAC 192-194 6 Declaration by the Head of Institution 195 7 Certificate of Compliance 196 Annexure I-VIII : 197-212 Annexure-I : Master Plan of the College 197 Annexure-II : Certificate of Recognition by Govt. -
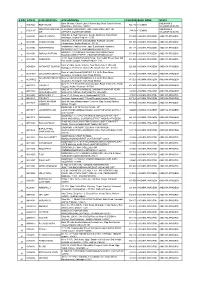
S No Atm Id Atm Location Atm Address Pincode Bank
S NO ATM ID ATM LOCATION ATM ADDRESS PINCODE BANK ZONE STATE Bank Of India, Church Lane, Phoenix Bay, Near Carmel School, ANDAMAN & ACE9022 PORT BLAIR 744 101 CHENNAI 1 Ward No.6, Port Blair - 744101 NICOBAR ISLANDS DOLYGUNJ,PORTBL ATR ROAD, PHARGOAN, DOLYGUNJ POST,OPP TO ANDAMAN & CCE8137 744103 CHENNAI 2 AIR AIRPORT, SOUTH ANDAMAN NICOBAR ISLANDS Shop No :2, Near Sai Xerox, Beside Medinova, Rajiv Road, AAX8001 ANANTHAPURA 515 001 ANDHRA PRADESH ANDHRA PRADESH 3 Anathapur, Andhra Pradesh - 5155 Shop No 2, Ammanna Setty Building, Kothavur Junction, ACV8001 CHODAVARAM 531 036 ANDHRA PRADESH ANDHRA PRADESH 4 Chodavaram, Andhra Pradesh - 53136 kiranashop 5 road junction ,opp. Sudarshana mandiram, ACV8002 NARSIPATNAM 531 116 ANDHRA PRADESH ANDHRA PRADESH 5 Narsipatnam 531116 visakhapatnam (dist)-531116 DO.NO 11-183,GOPALA PATNAM, MAIN ROAD NEAR ACV8003 GOPALA PATNAM 530 047 ANDHRA PRADESH ANDHRA PRADESH 6 NOOKALAMMA TEMPLE, VISAKHAPATNAM-530047 4-493, Near Bharat Petroliam Pump, Koti Reddy Street, Near Old ACY8001 CUDDAPPA 516 001 ANDHRA PRADESH ANDHRA PRADESH 7 Bus stand Cudappa, Andhra Pradesh- 5161 Bank of India, Guntur Branch, Door No.5-25-521, Main Rd, AGN9001 KOTHAPET GUNTUR 522 001 ANDHRA PRADESH ANDHRA PRADESH Kothapeta, P.B.No.66, Guntur (P), Dist.Guntur, AP - 522001. 8 Bank of India Branch,DOOR NO. 9-8-64,Sri Ram Nivas, AGW8001 GAJUWAKA BRANCH 530 026 ANDHRA PRADESH ANDHRA PRADESH 9 Gajuwaka, Anakapalle Main Road-530026 GAJUWAKA BRANCH Bank of India Branch,DOOR NO. 9-8-64,Sri Ram Nivas, AGW9002 530 026 ANDHRA PRADESH ANDHRA PRADESH -

MSEDCL Authority Padalkar Engineer Mail.Com Baramati Rural Circle, Urja Bhavan, II Floor, Public Information Keshav Vajanathrao Executive Sebaramati@G 1 7875768027
Baramati Zone Office,Baramati Sr. Office Name & First Appeallate Name of Assistant RTI Designation Mobile Number Email-Id No. Address officer / Public officer information officer/ Assistant Public information officer 1 Baramati Zone First Appeallate Sou. Poonam Ashish Superintending 7875768222 office officer Rokade Engineer baramati,Urjabh avan bhigwan road baramati Public information Uday Madhukar Kulkarni Executive 7875768028 officer Engineer sesatara@gmai l.com Assistant Public Dilipkumar Bajarang Deputy 7875768519 information officer Karvekar Executive Engineer Baramati Rural Circle Sr Office Name & First Appellate Name of Officer Designation in Mobile Number E-mail No Address Authority / Nodal Office Address Officer, Public Information Officer / System Administrator and Assistant Information Officer First Appellate Dattatraya Vishnu Superintending sebaramati@g 7875768111 MSEDCL Authority Padalkar Engineer mail.com Baramati Rural Circle, Urja Bhavan, II Floor, Public Information Keshav Vajanathrao Executive sebaramati@g 1 7875768027 Bhigwan road, Officer Kalumali Engineer mail.com Baramati, Pune- 413102 Assistant Information Dy Executive sebaramati@g Nilesh Ramling Borate 7875768081 Officer Engineer mail.com Baramati Division First Appellate Executive eebaramati@g Ganesh Manikrao Latpate 7875768005 Authority Engineer mail.com Baramati Division, Urjabhavan, I Public Information Dy Executive eebaramati@g 2 Floor, Bhigwan Savita Rahul Khatavkar 7875768096 Officer Engineer mail.com Road Baramati Tal : Baramati Dist : Pune Assistant Information -

Research Paper Commerce
Volume-4, Issue-4, April-2015 • ISSN No 2277 - 8160 Commerce Research Paper Geography Geographical Analysis of Shirurtahsil’s Literacy, Pune District, Ms Dr. Ratnaprabha Assist. Prof., C. T. Bora College, Shirur, Dist. Pune Santosh Jadhav ABSTRACT Literacy is one of the demographic characteristics which determines the rate of fertility, size of family, attitude about the female child, level of human resource development, socio-economic condition etc. It is the one of the determinants of eradicating poverty reduction, achieving gender equality and ensuring sustainable development. By taking into consideration the importance of literacy and its characteristics, an attempt has been made to analyze the circle-wise effective literacy,sex-wise literacy and literacy pattern in Shirur Tahsil of Pune District.The circle has been taken as a unit for analysis.For the detail analysis of literacy, 117 villages and 6 circles of this tahsil have been studiedby applying the 1991, 2001 and 2011census data from Town and Village Directory of Pune District. MS-Excel was applied to process and represent the data. ArcGIS was employed to prepare the base map and thematic maps of the study area. The study has observed that in Shirurtahsil, the effective literacy was recorded only 68.15 percent in 1991and it reached upto 82.37 percent in 2011. It showed 26 percent remarkable positive growth during the last two decades due to the development of educational facilities, transportation, agricultural and economic development etc. KEYWORDS : Literacy, literacy pattern, Effective Literacy and Sex-wise literacy. Introduction: six circles, namely Pabal, Shirur, Takali-Haji, TalegaonDhamdhere, Nha- Any person above the age of seven years, who can read and write in vara and Vadgaon-Rasai and covering 117 villages. -

Designing Place Topologies of Maker Labs
Designing Place Topologies of Maker Labs Sascha Rashof PhD Cultural Studies Centre for Cultural Studies Goldsmiths College I declare that the work presented in this thesis is my own. 2 Acknowledgements First of all, I would like to thank my (co-)supervisors Prof. Scott Lash, Dr. Luciana Parisi and Prof. Bernard Stiegler. I also want to thank the Arts and Humanities Research Council for funding this project for three years. I am grateful to my interviewees who generously donated their time, especially to Amitraj Deshmukh and his family for their assistance and care in India. Thanks to Matthew Holder for his support in academic as well as personal matters and for making life as enjoyable as it could be throughout writing up. I finally want to thank Dr. David Cunningham for his help in all things Heideggerian. 3 Abstract This PhD thesis is working towards a techno-social ontology of ‘place’. Place [topos] has been an underdeveloped concept in modern philosophical thought in ‘the West’, mainly subordinated to the more universal/ising terms time and space. Also media theory has often used conceptions of time as its primary category due to its foundation on notions of ‘process’. Even though media theory and geography are increasingly converging, however predominantly through conceptions of space, considerable ontological treatments of medial place are still missing. In order to develop a notion of place/s that is more singular and pluralistic than Deleuze and Guattari’s (spatial) rhizomes, which have now largely become the logics of post-Fordism, this thesis works with Peter Sloterdijk’s topo-logy of Spheres – through, with, beyond and against Heidegger’s ontology. -

Annual Report Annual Report
Annual Report 2011-12 Vigyan Ashram At.Post.Pabal Dist.Pune 412403 (A center of Indian Institute Of Education) 128/2, J.P Naik Path, Karve Road, Kothrud Pune 411038, Ph : 020-25424580 www.vigyanashram.com 1 Vigyan Ashram Annual Report 2011-12 Vigyan Ashram Annual Report 20112011----12121212 Financial year 2011-12 is marked with many landmarks for Vigyan Ashram. Most importantly Vigyan Ashram got new workshop facility with the financial support of M/S GKN Plc. and up gradation of food lab with the support of M/S Praj foundation. Important achievements and highlights of different projects of Vigyan Ashram are presented here. Vigyan Ashram is working on the philosophy of ‘Rural Development through Education System (RDES)’. Fig. 1 shows the model of RDES program. To achieve objective of rural development, VA has program, as shown in the fig. 2. Figure 1: RDES philosophy Figure 2: RDES Program 2 Vigyan Ashram Annual Report 2011-12 Following are the main program of Vigyan Ashram and major financial partners in the project (Table1). VIGYAN ASHRAM’S PROGRAM Technology Non formal Education Replication Program Infrastructural Development (DBRT & short term (Introduction to Basic Development at Pabal courses Program at Technology (IBT)) center Pabal) Program Supported by Dept of science and Asha for Education, E- Asha for education, GKN Sinter Metal, M/S Technology, INDUSA Boss, Honeywell, Lend-a-hand-India, Praj foundation, individual donor Suzlon foundation, Govt of Maharashtra, E- Hemendra Kothari BOSS (Mr.Meghaji) foundation, Govt of Chattisgarh, AID, We want to take this opportunity to thanks our donors, parents, students, villagers in Pabal and community for their continuous support and faith in Vigyan Ashram’s team. -

Vigyan Ashram Status Report
Vigyan Ashram Status Report Volume 38 Issue 08 August 2020 In this issue # Vigyan Ashram in Action # Under Lockdown • Independence Day & Ganesha Festival Celebration • Praj Foundation’s support to Rural Entrepreneurship Program • Fab School @ Mukhai School • DIC : Technology development updates • DBRT and IBT : Learn ‘Virtually’ and ‘Practice’ locally A] Independence Day & Ganesh festival celebration: 15 August Independence Day was celebrated with all Josh of celebration. Though we are missing students on campus due to pandemic. Participation by all Staff and DIC fellows made it a memorable day. There were cultural programs and sports activities. We had sport competition of relay race and underarm cricket matches. Cycle racing, limbu-chamcha (lemon spoon), Gunny sack racing were all fun. We also celebrated Ganesh festival during 21st to 26th August. VA team prepared Ganesh idol, made a small decoration & traditional ‘prasad’ during the festival. Staff also performed Barchhi dance with traditional Dhol and Tasha. It was full of fun & stress busters for team members. B] Entrepreneurship Development Program (EDP) : During past 5 months, more than 500 + rural youth, SHG members reached us through social media campaign, webinars & online training support. Presently we are providing incubation & handholding support to selected youth. Though pandemic & economic slowdown is negatively impacting rural community, many youth are willing to stay back in their villages. We are guiding them in selecting right opportunity & prepare business plan. Vishal & Ganesh visited Ahupe village (Tal-Ambegaon) on 7th Aug to survey skill training and livelihood needs of local youth. Ahupe is very remote village located at back water of Dhimbe dam. They are many reverse migrated youth in this village. -

Investor First Name Investor Middle Name Investor Last Name Father
Investor First Name Investor Middle Name Investor Last Name Father/Husband First Name Father/Husband Middle Name Father/Husband Last Name Address Country State District Pin Code Folio No. DP.ID-CL.ID. Account No. Invest Type Amount Transferred Proposed Date of Transfer to IEPF PAN Number Aadhar Number 74/153 GANDHI NAGAR A ARULMOZHI NA INDIA Tamil Nadu 636102 IN301774-10480786-0000 Amount for unclaimed and unpaid dividend 160.00 15-Sep-2019 ATTUR 1/26, VALLAL SEETHAKATHI SALAI A CHELLAPPA NA KILAKARAI (PO), INDIA Tamil Nadu 623517 12010900-00960311-TE00 Amount for unclaimed and unpaid dividend 60.00 15-Sep-2019 RAMANATHAPURAM KILAKARAI OLD NO E 109 NEW NO D A IRUDAYAM NA 6 DALMIA COLONY INDIA Tamil Nadu 621651 IN301637-40636357-0000 Amount for unclaimed and unpaid dividend 20.00 15-Sep-2019 KALAKUDI VIA LALGUDI OPP ANANDA PRINTERS I A J RAMACHANDRA JAYARAMACHAR STAGE DEVRAJ URS INDIA Karnataka 577201 IN300360-10245686-0000 Amount for unclaimed and unpaid dividend 8.00 15-Sep-2019 ACNPR4902M NAGAR SHIMOGA NEW NO.12 3RD CROSS STREET VADIVEL NAGAR A J VIJAYAKUMAR NA INDIA Tamil Nadu 632001 12010600-01683966-TE00 Amount for unclaimed and unpaid dividend 100.00 15-Sep-2019 SANKARAN PALAYAM VELLORE THIRUMANGALAM A M NIZAR NA OZHUKUPARAKKAL P O INDIA Kerala 691533 12023900-00295421-TE00 Amount for unclaimed and unpaid dividend 20.00 15-Sep-2019 AYUR AYUR FLAT - 503 SAI DATTA A MALLIKARJUNAPPA ANAGABHUSHANAPPA TOWERS RAMNAGAR INDIA Andhra Pradesh 515001 IN302863-10200863-0000 Amount for unclaimed and unpaid dividend 80.00 15-Sep-2019 AGYPA3274E -

Aquaponics (RAS) Trial 2.0 – Experience from Vigyan Ashram , Pabal (Pune, India)
Aquaponics (RAS) Trial 2.0 – Experience from Vigyan ashram , Pabal (Pune, India) Vigyan Ashram is working on aquaponics farming system from past 3 years. Aim is to standardize this system as per Indian agro-climatic conditions and market potentials. We conducted a fresh trial @ Vigyan ashram, Pabal, Tal- Shirur, Dist- Pune during September 2016 to May2017. This report covers details of trial with some of challenges faced during this period. Following were the tested aquaponics system components & trial details as - System components - A. Bio-Bed - Plastic paper (HDPE 250 GSM) lined bio-bed was constructed with approximate volume of 10000 lit (0.7 M depth , 2.5 width & 3.5 M length ) .Total of 5 grow beds constructed with cumulative volume of 6 M2. Elevation difference of 0.25 M was maintained in each bed so as to achieve siphon based water circulation. Beds were field with red mud bricks as growing media. Coco-pit filed grow beds were also placed in each bed as vegetable planting beds while Colocasia esculenta (Alu) , Solanum lycopersicum var. cerasiforme (Cherry tomato) , Brassica oleracea (Cauliflower), Capsicum annuum (Chilli) and Cucumis spp. (Cucumber) during trial period. B. Fish tank – Fish tank of with approximate 10000 lit capacity constructed with HDPE plastic paper linings. A drainage line was fixed at bottom of tank for removing fish / food waste. This drainage pipe was connected to sludge tank with floating valve for maintains of water level in tank. Water from fish tank is pumped to bio-bed & taken back to fish tank by with siphon based system. C. -

Bamboo As a Solution for Low-Cost Housing in India 1. Introduction
Lessons from India: Bamboo as a solution for low-cost housing in India R. Drake+, R. Morton#, C. Torres-Sánchez#1 + Department of Architecture and Civil Engineering, University of Bath, Bath, BA2 7AY # Department of Mechanical Engineering, Heriot-Watt University, Edinburgh, EH14 4AS Abstract This paper summarises the work done over a span of 18 months on bamboo as a structural material for low-cost housing solutions in Pabal, in the province of Pune, in India. The start of the project takes us to the University of Strathclyde in Glasgow, where the first designs and process methodology for building these DIY structures were conceived. After initial testing done in Scotland with 1:2 scale prototypes, a group from the University of Bath deployed in Ambajogai (Maharastra, India) built the full scale structure and reported back on the process and the difficulties encountered when erecting this structure in India along with the locals. The current work is focused on including the feedback obtained from the full scale construction, sorting out the major difficulties encountered, and adopting new solutions to counteract the setbacks that took place when the initial construction took place. Further work in the acoustics of the structures, as well as energy efficiency analyses are being carried out at present. The intention is to have another opportunity at building the new improved structure this coming summer, taking the advantage of EWB-affiliated students going to India for work placements or study trips. Keywords: Bamboo, Structure, Materials, Connections, Habitat, India 1. Introduction This work sees its continuity in the work carried out in three Higher Education UK Institutions, the University of Strathclyde, in Glasgow, the University of Bath, and Heriot-Watt University, in Edinburgh. -

District Census Handbook, Pune
CENSUS OF INDIA 1981 DISTRICT CENSUS HANDBOOK PUNE Compiled by THE MAHARASHTRA CENSUS DIRECTORATE BOMBAY PRINTED IN INDIA BY THE MANAGER, GOVERNMENT CENTRAL PRESS, BOMBAY AND PUBLISHED BY THE DIRECTOR, GOVERNMENT PRINTING, STATIONERY AND PUBLICATIONS, MAHARASHTRA STATE, BOMBAy-400 004. 1986 [Price Rs. 30.00] lLJ S c o "" « Z ;! ~a,'~,,_ ~ 0:: :::> ~ g ~ f -~, ~ Q. ~ 0 ~ ~ g :::r: ~ :z: ~ J- ~ § .! 0:: U <S ~ « ~ ::r: 0::: ~ ~ ~ « J- .j ~ 0 (J) ~ ~ ~ LO '5, ~ ~ :'! 'j' ~ 0 c- i '0 .g 02 ~ f:z: li ~~~ti<!::':ZI- It p. (', P. I'- \) <t po. a:: ~ ..(. <t I>- ,. .-~ .;>~<:> <t '- /'\ i ' " U'l C,; \. \ "- i I,~ lC ~1({:;OJ.j , ~. ' .~ ..... .'. a.~ u I/) a:: i' 0 " Ul (' <,' 0 ~/1·...... ·"r·j ,,r-./ C> .tV /& 1'-" , .IS 10 c, It 0- '\. ". ""• ~ :; ,F \. ') V ij c 0 ~ <Il~ MOTIF Shaniwar Wada, once upon a time was the palace of Peshwas (chief executives of the Maratha Kingdom founded by Chhatrapati Shivaji). Peshwas went on conquering places as far as Panipat and Atak and extending the boundaries of the kingdom beyond Chambal river in North India. This finest palace of the time (1730-1818) but now in dilapidated condition is in the heart of the Pune city. This palace as it finally stood was a seven-storeyed building with four large and several small courts some of which were named as Ganapati Rang Mahal, Nach Diwankhana, Arse Maltal, Hastidanti Mahal. Today, the remains (enclosure, plinths and the surrounding wall) are mute witnesses of the past Maratha glory. CENSUS OF INDIA 1981 SERIES 12-MAHARASHTRA DISTRICT PUNE ERRATA SLIP Page Column No Item No.