MIRE in BROADLAND, NORFOLK. By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Norfolk Local Flood Risk Management Strategy
Appendix A Norfolk Local Flood Risk Management Strategy Consultation Draft March 2015 1 Blank 2 Part One - Flooding and Flood Risk Management Contents PART ONE – FLOODING AND FLOOD RISK MANAGEMENT ..................... 5 1. Introduction ..................................................................................... 5 2 What Is Flooding? ........................................................................... 8 3. What is Flood Risk? ...................................................................... 10 4. What are the sources of flooding? ................................................ 13 5. Sources of Local Flood Risk ......................................................... 14 6. Sources of Strategic Flood Risk .................................................... 17 7. Flood Risk Management ............................................................... 19 8. Flood Risk Management Authorities ............................................. 22 PART TWO – FLOOD RISK IN NORFOLK .................................................. 30 9. Flood Risk in Norfolk ..................................................................... 30 Flood Risk in Your Area ................................................................ 39 10. Broadland District .......................................................................... 39 11. Breckland District .......................................................................... 45 12. Great Yarmouth Borough .............................................................. 51 13. Borough of King’s -

Canoe and Kayak Licence Requirements
Canoe and Kayak Licence Requirements Waterways & Environment Briefing Note On many waterways across the country a licence, day pass or similar is required. It is important all waterways users ensure they stay within the licensing requirements for the waters the use. Waterways licences are a legal requirement, but the funds raised enable navigation authorities to maintain the waterways, improve facilities for paddlers and secure the water environment. We have compiled this guide to give you as much information as possible regarding licensing arrangements around the country. We will endeavour to keep this as up to date as possible, but we always recommend you check the current situation on the waters you paddle. Which waters are covered under the British Canoeing licence agreements? The following waterways are included under British Canoeing’s licensing arrangements with navigation authorities: All Canal & River Trust Waterways - See www.canalrivertrust.org.uk for a list of all waterways managed by Canal & River Trust All Environment Agency managed waterways - Black Sluice Navigation; - River Ancholme; - River Cam (below Bottisham Lock); - River Glen; - River Great Ouse (below Kempston and the flood relief channel between the head sluice lock at Denver and the Tail sluice at Saddlebrow); - River Lark; - River Little Ouse (below Brandon Staunch); - River Medway – below Tonbridge; - River Nene – below Northampton; - River Stour (Suffolk) – below Brundon Mill, Sudbury; - River Thames – Cricklade Bridge to Teddington (including the Jubilee -

Broads Water Quality Report: River Thurne 2015
East Anglia Area (Essex, Norfolk and Suffolk) Broads water quality report: River Thurne 2015 Map showing the location of water quality sampling sites in the River Thurne and Broads. Broads water quality report: River Thurne 2015 Page 1 of 6 Status River: Water quality is good for dissolved oxygen, ammonia and nitrate. Broads: Nutrients (phosphorus and nitrogen – including ammonia and nitrate) are not all meeting the national and international standards High nutrient concentrations have a negative effect on the ecology of the broads. Nutrient sources include internal release from sediments, diffuse sources and tidal mixing of water from downstream. It is estimated that 97% of phosphorus in the Upper Thurne Broads and marshes comes from diffuse sources such as agriculture, minor point sources and septic tanks. Ammonia levels fail the water quality standards in Horsey Mere where concentrations are noticeably higher than the other broads in the Thurne. This is because there is an input of ammonia to the broad from the surface drains via Brograve drainage pump. The water in the Thurne river and broads is brackish. This is caused by sea water percolating through the ground close to the coast which is then drawn through drainage pumps into the broads and rivers. Actions The water catchment around the Thurne is designated as a Nitrate Vulnerable Zone. In this zone limits are set on when and how much nitrogen can be applied to agricultural land to reduce the amount of nitrate reaching the rivers and broads. https://www.gov.uk/guidance/nutrient-management-nitrate-vulnerable-zones Following studies done in 2014, the dominant source of ammonia in the Brograve drain is believed to be from agricultural activity. -

Martham Boats Vehicles
services About us DAY BOAT HIRE BEDDING All of our boats come with clean and laundered bedding ready to make regattas your stay as comfortable as possible. MARTHAM GARAGE ARRANGEMENTS At owner’s risk, cars may be garaged under cover at our works or parked outdoors for the period of your holiday. A charge is made for this. We strongly recommend customers to take advantage of the Why not hire one of our sailing boats undercover parking facility. BOATS TRANSPORT On arrival at either the train or coach station, telephone 01493 CLASSIC MOTOR & SAILING HOLIDAYS ON for the following events: 740249 and we shall be pleased to collect your party with one of the company Founded in 1946 and set in the very heart of the Norfolk Broads, Martham Boats vehicles. This extends to the return of your party on the day of departure. A is a traditional boat yard that hires traditional wooden cruisers, sailing yachts, THE NORFOLK BROADS small charge is made to cover the cost of fuel. half-deckers, bungalows, motor cruisers, canoes and day launches on the River Thurne. Horsey Mere REGATTA LUGGAGE Luggage forwarded in advance is accepted and stored until your motor • paddle • SAIL In 2013 Horsey Mere Regatta returned for the first time in over a century , arrival. Empty cases may be stored at the boatyard whilst you are on your cruise. Set in idyllic Norfolk Broads countryside,we offer a wide range of self catering attracting half deckers, production cruisers, hire yachts and dingies who take accommodation from cosy riverside bungalows to the hire of traditional hand part in several races over the weekend. -
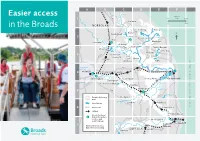
Easier Access Guide
A B C D E F R Ant Easier access A149 approx. 1 0 scale 4.3m R Bure Stalham 0 7km in the Broads NORFOLK A149 Hickling Horsey Barton Neatishead How Hill 2 Potter Heigham R Thurne Hoveton Horstead Martham Horning A1 062 Ludham Trinity Broads Wroxham Ormesby Rollesby 3 Cockshoot A1151 Ranworth Salhouse South Upton Walsham Filby R Wensum A47 R Bure Acle A47 4 Norwich Postwick Brundall R Yare Breydon Whitlingham Buckenham Berney Arms Water Gt Yarmouth Surlingham Rockland St Mary Cantley R Yare A146 Reedham 5 R Waveney A143 A12 Broads Authority Chedgrave area river/broad R Chet Loddon Haddiscoe 6 main road Somerleyton railway A143 Oulton Broad Broads National Park information centres and Worlingham yacht stations R Waveney Carlton Lowesto 7 Grid references (e.g. Marshes C2) refer to this map SUFFOLK Beccles Bungay A146 Welcome to People to help you Public transport the Broads National Park Broads Authority Buses Yare House, 62-64 Thorpe Road For all bus services in the Broads contact There’s something magical about water and Norwich NR1 1RY traveline 0871 200 2233 access is getting easier, with boats to suit 01603 610734 www.travelinesoutheast.org.uk all tastes, whether you want to sit back and www.broads-authority.gov.uk enjoy the ride or have a go yourself. www.VisitTheBroads.co.uk Trains If you prefer ‘dry’ land, easy access paths and From Norwich the Bittern Line goes north Broads National Park information centres boardwalks, many of which are on nature through Wroxham and the Wherry Lines go reserves, are often the best way to explore • Whitlingham Visitor Centre east to Great Yarmouth and Lowestoft. -

February 2010
1 THE EASTERLING JOURNAL OF THE EAST ANGLIAN WATERWAYS ASSOCIATION VOLUME EIGHT, NUMBER SIXTEEN FEBRUARY 2010 Edited by Alan H. Faulkner 43 Oaks Drive, Colchester, Essex CO3 3PS Phone 01206 767023 E-mail [email protected] A SORRY STATE OF AFFAIRS The IWA Peterborough Branch’s cruise for the Old Bedford had been planned for the weekend of Saturday 26 th September 2009. The entrance to the Old Bedford is via a tidal sluice, which can only be opened briefly when the tidal river Great Ouse is at low tide. Back in 1973, when the Old Bedford was the only open route through the Middle Level for those attending the IWA’s Ely Rally, over 40 boats were able to use the sluice on the one tide. Today, the silting of the Great Ouse below Denver has meant that it is over a year since a passage had been attempted. During the previous week the Environment Agency had brought a floating Smalley excavator and a land-based dredger, to open out the entrance into the Old Bedford, and the channel sides were re-profiled. To “test” the work on the Friday, the narrow boat cruiser OLIVE EMILY had locked out of Salter’s Lode Lock (Well Creek) just before low tide and attempted to enter the mouth of the Old Bedford, but unfortunately not enough silt had been dredged from below the waterline for the boat to enter. Not wishing to miss the “window” of opening of the tidal doors John Revell, the boater, decided to reverse in, using his propeller to carve out his own channel, and he managed to reach the Old Bedford. -

Planning for Waterside Bungalows/Chalets
Planning for waterside bungalows/chalets Bure at Hoveton Planning for waterside bungalows/chalets This guide outlines the history of Early tourists waterside bungalow/chalets and the contribution they make within the Waterside bungalow/chalets are part of Broads, discusses their similarities the unique Broads landscape. Most of and differences, and suggests ways to the bungalow/chalets we see today stem maintain and alter existing bungalow/ from holidaymaking in the Broads from chalets and insert new bungalow/chalets the 1880s to the 1960s. They are a distinct successfully within their particular group of buildings which significantly historic and landscape setting. For contribute to our understanding of the the purposes of this guide, the term history of the Broads. In the late 1800s, Bungalow/chalet relates to small/ if you had some disposable income, what low light-weight buildings which are better way to dispose of it than on a generally at the water’s edge. waterside bungalow/chalet in the Broads? Waterside bungalow/chalets were initially Part 1: Changing perceptions built for this expanding holiday market consisting mainly of affluent city dwellers Waterside bungalow/chalets undoubtedly who sought refuge within the wild and make an impact on the character of the undeveloped Broads in the late 19th and riverbank. Historically there was concern early 20th century. The growth of tourism that in some locations this was starting in the Broads was closely linked to the to become negative. For example back in establishment of railway stations within 1982 the Broads Authority was keen to the Victorian period and some of the most remove some of the bungalow/chalets on popular areas for waterside bungalow/ the River Thurne at Potter Heigham and chalets were around villages with links to Martham. -

Wroxham Broad to Barton Broad Wroxham to Ant Mouth the To
Explore the Broads 2021 Saturday - Wroxham Broad to Barton Broad Wroxham to Ant Mouth The tour starts at the Norfolk Broads Yacht Club on Wroxham Broad. The first leg is relatively short and the river Bure is relatively wide, so a relaxed start to the tour. Leave the Yacht Club and aim for the opening on to the River Bure on the far right-hand side of the broad between posts marked 1 and 2. Keep on the main river unless you wish to explore Salhouse Broad which is off to the right just past some moorings on the on the left. Above is the entrance and the exit is marked by red posts. Follow the river to Horning for lunch at The Swan Inn. A space for us to take the boats out will have been kept clear to the left of the pub and to the right of Horning Sailing Club. Beware that the ‘Mississippi paddle boat’ may be moored by this grassed area as well. After lunch, launch the boat and continue along the river in the same direction. Look out for a sign to the mouth of the River Ant on the left-hand side of the river just past Horning Hall and turn left. Explore the Broads 2021 Page 1 of 4 Ant Mouth to Barton Broad Turn left off the River Bure The River Ant is narrower and winds through high reed beds. As you approach Ludham Bridge there are tight bends with boats moored on both sides. Immediately round the bends is the bridge – narrow but manageable with care. -

BA 2018 0514 FUL Ludham Field Base Report by Planning Officer
Planning Committee 10 January 2020 Agenda item number 8 BA 2018 0514 FUL Ludham Field base Report by Planning Officer Proposal Extension of mooring basin and realignment of access from Womack Dyke. Applicant Mr Paul Carrington Recommendation Approval subject to conditions and s106 agreement Reason for referral to committee Objections from internal consultees Application target date 12 March 2019 Contents 1. Description of site and proposals 2 2. Site history 3 3. Consultations received 3 4. Representations 5 5. Policies 5 6. Assessment 6 7. Conclusion 12 8. Recommendation 13 9. Reason for recommendation 13 Appendix 1 - Location map 14 Planning Committee, 10 January 2020, agenda item number 8 1 1. Description of site and proposals 1.1. The subject site comprises a mooring basin and adjacent land at the Ludham Field Base Centre sited on the north-east side of Womack Water at the far south-eastern end of the village of Ludham. The subject mooring basin serves the workshop and offices of the former Broads Authority field base, currently the home of the Norfolk Broads Yachting Company, accessed from Horsefen Road. The wider site also provides an area of car parking and associated hardstanding. 1.2. The area at the southern end of Horsefen Road and fronting Womack Water is home to a small cluster of boat workshops and mooring basins. To the immediate east of the subject site is Hunters Yard, home to the Norfolk Heritage Fleet Trust. To the immediate west is the Forsythe Wherry Yard, home to the Norfolk Wherry Trust, a site which includes a boathouse utilised by the Broads Authority as a billet for a launch, and beyond that is the Swallowtail Boatyard. -

Broads Water Quality Report: River Thurne 2015
East Anglia Area (Essex, Norfolk and Suffolk) Broads water quality report: River Thurne 2015 Broads Forum 2 February 2017 Agenda Item 6 Map showing the location of water quality sampling sites in the River Thurne and Broads. Broads water quality report: River Thurne 2015 Page 1 of 6 Status River: Water quality is good for dissolved oxygen, ammonia and nitrate. Broads: Nutrients (phosphorus and nitrogen – including ammonia and nitrate) are not all meeting the national and international standards High nutrient concentrations have a negative effect on the ecology of the broads. Nutrient sources include internal release from sediments, diffuse sources and tidal mixing of water from downstream. It is estimated that 97% of phosphorus in the Upper Thurne Broads and marshes comes from diffuse sources such as agriculture, minor point sources and septic tanks. Ammonia levels fail the water quality standards in Horsey Mere where concentrations are noticeably higher than the other broads in the Thurne. This is because there is an input of ammonia to the broad from the surface drains via Brograve drainage pump. The water in the Thurne river and broads is brackish. This is caused by sea water percolating through the ground close to the coast which is then drawn through drainage pumps into the broads and rivers. Actions The water catchment around the Thurne is designated as a Nitrate Vulnerable Zone. In this zone limits are set on when and how much nitrogen can be applied to agricultural land to reduce the amount of nitrate reaching the rivers and broads. https://www.gov.uk/guidance/nutrient-management-nitrate-vulnerable-zones Following studies done in 2014, the dominant source of ammonia in the Brograve drain is believed to be from agricultural activity. -

Anglian River Basin District Flood Risk Management Plan 2015 - 2021 PART B – Sub Areas in the Anglian River Basin District
Anglian River Basin District Flood Risk Management Plan 2015 - 2021 PART B – Sub Areas in the Anglian River Basin District March 2016 1 of 161 Published by: Environment Agency Further copies of this report are available Horizon house, Deanery Road, from our publications catalogue: Bristol BS1 5AH www.gov.uk/government/publications Email: [email protected] Or the Environment Agency’s National www.gov.uk/environment-agency Customer Contact Centre: T: 03708 506506 © Environment Agency 2016 Email: [email protected]. All rights reserved. This document may be reproduced with prior permission of the Environment Agency. 2 of 161 Contents Glossary and abbreviations ......................................................................................................... 5 The layout of this document ........................................................................................................ 7 1. Sub-areas in the Anglian River Basin District ...................................................................... 9 Introduction ................................................................................................................................... 9 Flood Risk Areas ........................................................................................................................ 10 Management Catchments ........................................................................................................... 10 2. Conclusions, objectives and measures to manage risk in the South Essex -

Tactical Flood Response Plan Part One
OFFICIAL Tactical Flood Response Plan Part One Version 5.1 Author NRF Severe Weather & Flood Risk Group Reviewed by NRF Severe Weather & Flood Risk Authorised by Environment Agency Next review date June 2018 OFFICIAL Page 1 of 65 OFFICIAL Foreword This document has been produced after consultation with Category 1 and 2 Responders (as defined within the Civil Contingencies Act 2004), through the Norfolk Resilience Forum. It provides guidance by which Norfolk can be suitably prepared to respond to an actual or potential major flooding emergency, whereby the combined resources of numerous agencies are required. It will be used by these agencies when information is received or events occur that require a coordinated response at the tactical level. Tom McCabe NRF Executive Lead – Protection Capability Workstream Norfolk County Council OFFICIAL Page 2 of 65 OFFICIAL Table of Contents Foreword .............................................................................................................................................................................................2 Purpose ...............................................................................................................................................................................................8 Local Considerations: ........................................................................................................................................................................8 Protocols .............................................................................................................................................................................................9