Wsn 99 (2018) 193-214 Eissn 2392-2192
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fauna Lepidopterologica Volgo-Uralensis" 150 Years Later: Changes and Additions
©Ges. zur Förderung d. Erforschung von Insektenwanderungen e.V. München, download unter www.zobodat.at Atalanta (August 2000) 31 (1/2):327-367< Würzburg, ISSN 0171-0079 "Fauna lepidopterologica Volgo-Uralensis" 150 years later: changes and additions. Part 5. Noctuidae (Insecto, Lepidoptera) by Vasily V. A n ik in , Sergey A. Sachkov , Va d im V. Z o lo t u h in & A n drey V. Sv ir id o v received 24.II.2000 Summary: 630 species of the Noctuidae are listed for the modern Volgo-Ural fauna. 2 species [Mesapamea hedeni Graeser and Amphidrina amurensis Staudinger ) are noted from Europe for the first time and one more— Nycteola siculana Fuchs —from Russia. 3 species ( Catocala optata Godart , Helicoverpa obsoleta Fabricius , Pseudohadena minuta Pungeler ) are deleted from the list. Supposedly they were either erroneously determinated or incorrect noted from the region under consideration since Eversmann 's work. 289 species are recorded from the re gion in addition to Eversmann 's list. This paper is the fifth in a series of publications1 dealing with the composition of the pres ent-day fauna of noctuid-moths in the Middle Volga and the south-western Cisurals. This re gion comprises the administrative divisions of the Astrakhan, Volgograd, Saratov, Samara, Uljanovsk, Orenburg, Uralsk and Atyraus (= Gurjev) Districts, together with Tataria and Bash kiria. As was accepted in the first part of this series, only material reliably labelled, and cover ing the last 20 years was used for this study. The main collections are those of the authors: V. A n i k i n (Saratov and Volgograd Districts), S. -

Insektenkasten Mit Eulenfaltern
Insektenkasten mit Eulenfaltern Kasten mit verschiedenen Arten von Eulenfaltern (Noctuidae) Maße: 40 x 30 x 6 cm Inventarnummer: ohne Aus der Insektensammlung des Departments für Evolutionsbiologie „Der erste Grad der Weisheit, sagt der Ritter v. Linné [1767, p. 12], ist das Erkenntniß der Dinge. Dieses Erkenntniß besteht in einem wahren Begriffe der Gegenstände, vermöge dessen Aehnliche von Unähnlichen durch eigene vom Schöpfer ihnen aufgedrückte Kennzeichen unterschieden werden. Wer dieses Kenntniß andern mittheilen will, muß allen verschiedenen Dingen eigene Namen geben, die niemal vermenget werden müßen. Denn mit den Namen fällt auch das Erkenntniß der Dinge.“ (Denis & Schiffermüller 1776, p. 23) Diese Feststellung Carl von Linnés (1707–1778), getroffen in der zwölften Auflage seines Hauptwerkes Systema naturae, fand als zentraler Gedanke Eingang in ein für die europäische Schmetterlingskunde grundlegendes Werk: das von Michael Denis (1729– 1800) und Ignaz Schiffermüller (1727–1806) verfasste „Systematische Verzeichniß der Schmetterlinge der Wienergegend“ (1 Aufl. 1775, 2 Aufl. 1776). Dieses Werk stellt eines der frühesten, mit dem Bemühen um Vollständigkeit angelegten Werke zur Schmetterlingsfauna eines geographisch klar umrissenen Gebietes dar. Das „Nebenprodukt“ dieser umfassenden Bestandsaufnahme war die Beschreibung einer sehr großen Zahl von damals der Wissenschaft noch unbekannten Arten aus Wien und Umgebung. Als Ausgangspunkt für ihre Bearbeitung diente den beiden Jesuiten Denis und Schiffermüller – beide Lehrer am Wiener Theresianum – eine umfangreiche selbst angelegte Schmetterlingssammlung. Auch an der Universität Wien wurde im darauffolgenden 19. Jahrhundert damit begonnen, eine Schmetterlingssammlung anzulegen (Salvini-Plawen & Mizzaro 1999, Waitzbauer 2012), die – so wie auch schon die Sammlung von Denis und Schiffermüller und ganz im Sinne des eingangs zitierten Linnés – primär als Lehrsammlung zur Vermittlung von Arten- und Formenkenntnis konzipiert war. -

Drepanidae (Lepidoptera)
ISSN: 1989-6581 Fernández Vidal (2017) www.aegaweb.com/arquivos_entomoloxicos ARQUIVOS ENTOMOLÓXICOS, 17: 151-158 ARTIGO / ARTÍCULO / ARTICLE Lepidópteros de O Courel (Lugo, Galicia, España, N.O. Península Ibérica) VII: Drepanidae (Lepidoptera). Eliseo H. Fernández Vidal Plaza de Zalaeta, 2, 5ºA. E-15002 A Coruña (ESPAÑA). e-mail: [email protected] Resumen: Se elabora un listado comentado y puesto al día de los Drepanidae (Lepidoptera) presentes en O Courel (Lugo, Galicia, España, N.O. Península Ibérica), recopilando los datos bibliográficos existentes (sólo para dos especies) a los que se añaden otros nuevos como resultado del trabajo de campo del autor alcanzando un total de 13 especies. Entre los nuevos registros aportados se incluyen tres primeras citas para la provincia de Lugo: Drepana curvatula (Borkhausen, 1790), Watsonalla binaria (Hufnagel, 1767) y Cimatophorina diluta ([Denis & Schiffermüller], 1775). Incluimos también nuevas citas de Drepanidae para otras localidades del resto del territorio gallego, entre las que aportamos las primeras de Falcaria lacertinaria (Linnaeus, 1758) para las provincias de Ourense y Pontevedra. Palabras clave: Lepidoptera, Drepanidae, O Courel, Lugo, Galicia, España, N.O. Península Ibérica. Abstract: Lepidoptera from O Courel (Lugo, Galicia, Spain, NW Iberian Peninsula) VII: Drepanidae (Lepidoptera). An updated and annotated list of the Drepanidae (Lepidoptera) know to occur in O Courel (Lugo, Galicia, Spain, NW Iberian Peninsula) is made, compiling the existing bibliographic records (only for two species) and reaching up to 13 species after adding new ones as a result of field work undertaken by the author. Amongst the new data the first records of Drepana curvatula (Borkhausen, 1790), Watsonalla binaria (Hufnagel, 1767) and Cimatophorina diluta ([Denis & Schiffermüller], 1775) for the province of Lugo are reported. -

Lepidoptera in Cheshire in 2002
Lepidoptera in Cheshire in 2002 A Report on the Micro-Moths, Butterflies and Macro-Moths of VC58 S.H. Hind, S. McWilliam, B.T. Shaw, S. Farrell and A. Wander Lancashire & Cheshire Entomological Society November 2003 1 1. Introduction Welcome to the 2002 report on lepidoptera in VC58 (Cheshire). This is the second report to appear in 2003 and follows on from the release of the 2001 version earlier this year. Hopefully we are now on course to return to an annual report, with the 2003 report planned for the middle of next year. Plans for the ‘Atlas of Lepidoptera in VC58’ continue apace. We had hoped to produce a further update to the Atlas but this report is already quite a large document. We will, therefore produce a supplementary report on the Pug Moths recorded in VC58 sometime in early 2004, hopefully in time to be sent out with the next newsletter. As usual, we have produced a combined report covering micro-moths, macro- moths and butterflies, rather than separate reports on all three groups. Doubtless observers will turn first to the group they are most interested in, but please take the time to read the other sections. Hopefully you will find something of interest. Many thanks to all recorders who have already submitted records for 2002. Without your efforts this report would not be possible. Please keep the records coming! This request also most definitely applies to recorders who have not sent in records for 2002 or even earlier. It is never too late to send in historic records as they will all be included within the above-mentioned Atlas when this is produced. -

Nota Lepidopterologica
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Nota lepidopterologica Jahr/Year: 2006 Band/Volume: 29 Autor(en)/Author(s): Fibiger Michael, Sammut Paul M., Seguna Anthony, Catania Aldo Artikel/Article: Recent records of Noctuidae from Malta, with five species new to the European fauna, and a new subspecies 193-213 ©Societas Europaea Lepidopterologica; download unter http://www.biodiversitylibrary.org/ und www.zobodat.at Notalepid. 29(3/4): 193-213 193 Recent records of Noctuidae from Malta, with five species new to the European fauna, and a new subspecies Michael Fibiger Paul Sammut-, Anthony Seguna \ & Aldo Catania^ ' Molbecha Allé 49, 4180 Sor0, Denmark; e-mail: [email protected] 2 137, 'Fawkner/2\ Dingli Rd., Rabat, RBT 07, Malta; e-mail: [email protected] ^ 'Redeemer', Triq 1-Emigrant, Naxxar, Malta; e-mail: [email protected] ^ 'Rama Rama', Triq Möns. Anton Cilia, Zebbug, Malta; e-mail: [email protected] Abstract. Recent records of Noctuoidea from Malta are given. Five noctuid species are recorded from Europe for the first time: Eublemma conistrota Hampson, 1910, Eiiblemma deserti Rothschild, 1909, Anumeta hilgerti (Rothschild 1909), Hadiila deserticula (Hampson 1905), and Eiixoa canariensis Rebel, 1902. New synonyms are stated: Leptosia velocissima f. tarda Turati, 1926, syn. n. and Leptosia griseimargo Warren, 1912, syn. n., both synonyms of Metachrostis velox (Hübner, 1813); and Pseudohadena (Eremohadena) roseonitens espugnensis Lajonquiere, 1964, syn. n., a synonym of P. (E.) roseonitens roseonitens (Oberthür, 1887). A new subspecies of Xylena exsoleta (Linneaus, 1758), Xylena exsoleta maltensis ssp. n., is established. The literature on Maltese Noctuoidea is reviewed and erronuousely reported species are indicated. -

Recerca I Territori V12 B (002)(1).Pdf
Butterfly and moths in l’Empordà and their response to global change Recerca i territori Volume 12 NUMBER 12 / SEPTEMBER 2020 Edition Graphic design Càtedra d’Ecosistemes Litorals Mediterranis Mostra Comunicació Parc Natural del Montgrí, les Illes Medes i el Baix Ter Museu de la Mediterrània Printing Gràfiques Agustí Coordinadors of the volume Constantí Stefanescu, Tristan Lafranchis ISSN: 2013-5939 Dipòsit legal: GI 896-2020 “Recerca i Territori” Collection Coordinator Printed on recycled paper Cyclus print Xavier Quintana With the support of: Summary Foreword ......................................................................................................................................................................................................... 7 Xavier Quintana Butterflies of the Montgrí-Baix Ter region ................................................................................................................. 11 Tristan Lafranchis Moths of the Montgrí-Baix Ter region ............................................................................................................................31 Tristan Lafranchis The dispersion of Lepidoptera in the Montgrí-Baix Ter region ...........................................................51 Tristan Lafranchis Three decades of butterfly monitoring at El Cortalet ...................................................................................69 (Aiguamolls de l’Empordà Natural Park) Constantí Stefanescu Effects of abandonment and restoration in Mediterranean meadows .......................................87 -
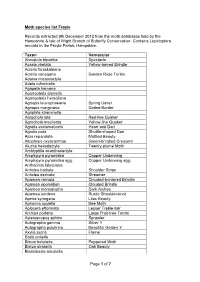
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

MOTHS and BUTTERFLIES LEPIDOPTERA DISTRIBUTION DATA SOURCES (LEPIDOPTERA) * Detailed Distributional Information Has Been J.D
MOTHS AND BUTTERFLIES LEPIDOPTERA DISTRIBUTION DATA SOURCES (LEPIDOPTERA) * Detailed distributional information has been J.D. Lafontaine published for only a few groups of Lepidoptera in western Biological Resources Program, Agriculture and Agri-food Canada. Scott (1986) gives good distribution maps for Canada butterflies in North America but these are generalized shade Central Experimental Farm Ottawa, Ontario K1A 0C6 maps that give no detail within the Montane Cordillera Ecozone. A series of memoirs on the Inchworms (family and Geometridae) of Canada by McGuffin (1967, 1972, 1977, 1981, 1987) and Bolte (1990) cover about 3/4 of the Canadian J.T. Troubridge fauna and include dot maps for most species. A long term project on the “Forest Lepidoptera of Canada” resulted in a Pacific Agri-Food Research Centre (Agassiz) four volume series on Lepidoptera that feed on trees in Agriculture and Agri-Food Canada Canada and these also give dot maps for most species Box 1000, Agassiz, B.C. V0M 1A0 (McGugan, 1958; Prentice, 1962, 1963, 1965). Dot maps for three groups of Cutworm Moths (Family Noctuidae): the subfamily Plusiinae (Lafontaine and Poole, 1991), the subfamilies Cuculliinae and Psaphidinae (Poole, 1995), and ABSTRACT the tribe Noctuini (subfamily Noctuinae) (Lafontaine, 1998) have also been published. Most fascicles in The Moths of The Montane Cordillera Ecozone of British Columbia America North of Mexico series (e.g. Ferguson, 1971-72, and southwestern Alberta supports a diverse fauna with over 1978; Franclemont, 1973; Hodges, 1971, 1986; Lafontaine, 2,000 species of butterflies and moths (Order Lepidoptera) 1987; Munroe, 1972-74, 1976; Neunzig, 1986, 1990, 1997) recorded to date. -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe. -

Monitoring Report Spring/Summer 2015 Contents
Wimbledon and Putney Commons Monitoring Report Spring/Summer 2015 Contents CONTEXT 1 A. SYSTEMATIC RECORDING 3 METHODS 3 OUTCOMES 6 REFLECTIONS AND RECOMMENDATIONS 18 B. BIOBLITZ 19 REFLECTIONS AND LESSONS LEARNT 21 C. REFERENCES 22 LIST OF FIGURES Figure 1 Location of The Plain on Wimbledon and Putney Commons 2 Figure 2 Experimental Reptile Refuge near the Junction of Centre Path and Somerset Ride 5 Figure 3 Contrasting Cut and Uncut Areas in the Conservation Zone of The Plain, Spring 2015 6/7 Figure 4 Notable Plant Species Recorded on The Plain, Summer 2015 8 Figure 5 Meadow Brown and white Admiral Butterflies 14 Figure 6 Hairy Dragonfly and Willow Emerald Damselfly 14 Figure 7 The BioBlitz Route 15 Figure 8 Vestal and European Corn-borer moths 16 LIST OF TABLES Table 1 Mowing Dates for the Conservation Area of The Plain 3 Table 2 Dates for General Observational Records of The Plain, 2015 10 Table 3 Birds of The Plain, Spring - Summer 2015 11 Table 4 Summary of Insect Recording in 2015 12/13 Table 5 Rare Beetles Living in the Vicinity of The Plain 15 LIST OF APPENDICES A1 The Wildlife and Conservation Forum and Volunteer Recorders 23 A2 Sward Height Data Spring 2015 24 A3 Floral Records for The Plain : Wimbledon and Putney Commons 2015 26 A4 The Plain Spring and Summer 2015 – John Weir’s General Reports 30 A5 a Birds on The Plain March to September 2015; 41 B Birds on The Plain - summary of frequencies 42 A6 ai Butterflies on The Plain (DW) 43 aii Butterfly long-term transect including The Plain (SR) 44 aiii New woodland butterfly transect -

The Entomologist's Record and Journal of Variation
M DC, — _ CO ^. E CO iliSNrNVINOSHilWS' S3ldVyan~LIBRARlES*"SMITHS0N!AN~lNSTITUTl0N N' oCO z to Z (/>*Z COZ ^RIES SMITHSONIAN_INSTITUTlON NOIiniIiSNI_NVINOSHllWS S3ldVaan_L: iiiSNi'^NviNOSHiiNS S3iavyan libraries Smithsonian institution N( — > Z r- 2 r" Z 2to LI ^R I ES^'SMITHSONIAN INSTITUTlON'"NOIini!iSNI~NVINOSHilVMS' S3 I b VM 8 11 w </» z z z n g ^^ liiiSNi NviNOSHims S3iyvyan libraries Smithsonian institution N' 2><^ =: to =: t/J t/i </> Z _J Z -I ARIES SMITHSONIAN INSTITUTION NOIiniliSNI NVINOSHilWS SSIdVyan L — — </> — to >'. ± CO uiiSNi NViNosHiiws S3iyvaan libraries Smithsonian institution n CO <fi Z "ZL ~,f. 2 .V ^ oCO 0r Vo^^c>/ - -^^r- - 2 ^ > ^^^^— i ^ > CO z to * z to * z ARIES SMITHSONIAN INSTITUTION NOIinillSNl NVINOSHllWS S3iaVdan L to 2 ^ '^ ^ z "^ O v.- - NiOmst^liS^> Q Z * -J Z I ID DAD I re CH^ITUCnMIAM IMOTtTIITinM / c. — t" — (/) \ Z fj. Nl NVINOSHIIINS S3 I M Vd I 8 H L B R AR I ES, SMITHSONlAN~INSTITUTION NOIlfl :S^SMITHS0NIAN_ INSTITUTION N0liniliSNI__NIVIN0SHillMs'^S3 I 8 VM 8 nf LI B R, ^Jl"!NVINOSHimS^S3iavyan"'LIBRARIES^SMITHS0NIAN~'lNSTITUTI0N^NOIin L '~^' ^ [I ^ d 2 OJ .^ . ° /<SS^ CD /<dSi^ 2 .^^^. ro /l^2l^!^ 2 /<^ > ^'^^ ^ ..... ^ - m x^^osvAVix ^' m S SMITHSONIAN INSTITUTION — NOIlfliliSNrNVINOSHimS^SS iyvyan~LIBR/ S "^ ^ ^ c/> z 2 O _ Xto Iz JI_NVIN0SH1I1/MS^S3 I a Vd a n^LI B RAR I ES'^SMITHSONIAN JNSTITUTION "^NOlin Z -I 2 _j 2 _j S SMITHSONIAN INSTITUTION NOIinillSNI NVINOSHilWS S3iyVaan LI BR/ 2: r- — 2 r- z NVINOSHiltNS ^1 S3 I MVy I 8 n~L B R AR I Es'^SMITHSONIAN'iNSTITUTIOn'^ NOlin ^^^>^ CO z w • z i ^^ > ^ s smithsonian_institution NoiiniiiSNi to NviNosHiiws'^ss I dVH a n^Li br; <n / .* -5^ \^A DO « ^\t PUBLISHED BI-MONTHLY ENTOMOLOGIST'S RECORD AND Journal of Variation Edited by P.A. -

Diversity of the Moth Fauna (Lepidoptera: Heterocera) of a Wetland Forest: a Case Study from Motovun Forest, Istria, Croatia
PERIODICUM BIOLOGORUM UDC 57:61 VOL. 117, No 3, 399–414, 2015 CODEN PDBIAD DOI: 10.18054/pb.2015.117.3.2945 ISSN 0031-5362 original research article Diversity of the moth fauna (Lepidoptera: Heterocera) of a wetland forest: A case study from Motovun forest, Istria, Croatia Abstract TONI KOREN1 KAJA VUKOTIĆ2 Background and Purpose: The Motovun forest located in the Mirna MITJA ČRNE3 river valley, central Istria, Croatia is one of the last lowland floodplain 1 Croatian Herpetological Society – Hyla, forests remaining in the Mediterranean area. Lipovac I. n. 7, 10000 Zagreb Materials and Methods: Between 2011 and 2014 lepidopterological 2 Biodiva – Conservation Biologist Society, research was carried out on 14 sampling sites in the area of Motovun forest. Kettejeva 1, 6000 Koper, Slovenia The moth fauna was surveyed using standard light traps tents. 3 Biodiva – Conservation Biologist Society, Results and Conclusions: Altogether 403 moth species were recorded Kettejeva 1, 6000 Koper, Slovenia in the area, of which 65 can be considered at least partially hygrophilous. These results list the Motovun forest as one of the best surveyed regions in Correspondence: Toni Koren Croatia in respect of the moth fauna. The current study is the first of its kind [email protected] for the area and an important contribution to the knowledge of moth fauna of the Istria region, and also for Croatia in general. Key words: floodplain forest, wetland moth species INTRODUCTION uring the past 150 years, over 300 papers concerning the moths Dand butterflies of Croatia have been published (e.g. 1, 2, 3, 4, 5, 6, 7, 8).