Tripartite Interactions Among Ixodiphagus Hookeri, Ixodes Ricinus and Deer: Differential Interference with Transmission Cycles of Tick-Borne Pathogens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Arthropod Pathogen Vectors in North America: Minimizing Adverse Effects on Pollinators
Journal of Medical Entomology, 2017, 1–13 doi: 10.1093/jme/tjx146 Forum Forum Management of Arthropod Pathogen Vectors in North America: Minimizing Adverse Effects on Pollinators Howard S. Ginsberg,1,2 Timothy A. Bargar,3 Michelle L. Hladik,4 and Charles Lubelczyk5 1USGS Patuxent Wildlife Research Center, University of Rhode Island, RI Field Station, Woodward Hall – PSE, Kingston, RI 02881 ([email protected]), 2Corresponding author, e-mail: [email protected], 3USGS Wetland and Aquatic Research Center, 7920 NW 71st St., Gainesville, FL 32653 ([email protected]), 4USGS California Water Science Center, 6000 J St., Placer Hall, Sacramento, CA 95819 ([email protected]), and 5Maine Medical Center Research Institute, Vector-Borne Disease Laboratory, 81 Research Dr., Scarborough, ME 04074 ([email protected]) Subject Editor: Lars Eisen Received 26 April 2017; Editorial decision 19 June 2017 Abstract Tick and mosquito management is important to public health protection. At the same time, growing concerns about declines of pollinator species raise the question of whether vector control practices might affect pollinator populations. We report the results of a task force of the North American Pollinator Protection Campaign (NAPPC) that examined potential effects of vector management practices on pollinators, and how these pro- grams could be adjusted to minimize negative effects on pollinating species. The main types of vector control practices that might affect pollinators are landscape manipulation, biocontrol, and pesticide applications. Some current practices already minimize effects of vector control on pollinators (e.g., short-lived pesticides and application-targeting technologies). Nontarget effects can be further diminished by taking pollinator protection into account in the planning stages of vector management programs. -

Parazitologický Ústav SAV Správa O
Parazitologický ústav SAV Správa o činnosti organizácie SAV za rok 2014 Košice január 2015 Obsah osnovy Správy o činnosti organizácie SAV za rok 2014 1. Základné údaje o organizácii 2. Vedecká činnosť 3. Doktorandské štúdium, iná pedagogická činnosť a budovanie ľudských zdrojov pre vedu a techniku 4. Medzinárodná vedecká spolupráca 5. Vedná politika 6. Spolupráca s VŠ a inými subjektmi v oblasti vedy a techniky 7. Spolupráca s aplikačnou a hospodárskou sférou 8. Aktivity pre Národnú radu SR, vládu SR, ústredné orgány štátnej správy SR a iné organizácie 9. Vedecko-organizačné a popularizačné aktivity 10. Činnosť knižnično-informačného pracoviska 11. Aktivity v orgánoch SAV 12. Hospodárenie organizácie 13. Nadácie a fondy pri organizácii SAV 14. Iné významné činnosti organizácie SAV 15. Vyznamenania, ocenenia a ceny udelené pracovníkom organizácie SAV 16. Poskytovanie informácií v súlade so zákonom o slobodnom prístupe k informáciám 17. Problémy a podnety pre činnosť SAV PRÍLOHY A Zoznam zamestnancov a doktorandov organizácie k 31.12.2014 B Projekty riešené v organizácii C Publikačná činnosť organizácie D Údaje o pedagogickej činnosti organizácie E Medzinárodná mobilita organizácie Správa o činnosti organizácie SAV 1. Základné údaje o organizácii 1.1. Kontaktné údaje Názov: Parazitologický ústav SAV Riaditeľ: doc. MVDr. Branislav Peťko, DrSc. Zástupca riaditeľa: doc. RNDr. Ingrid Papajová, PhD. Vedecký tajomník: RNDr. Marta Špakulová, DrSc. Predseda vedeckej rady: RNDr. Ivica Hromadová, CSc. Člen snemu SAV: RNDr. Vladimíra Hanzelová, DrSc. Adresa: Hlinkova 3, 040 01 Košice http://www.saske.sk/pau Tel.: 055/6331411-13 Fax: 055/6331414 E-mail: [email protected] Názvy a adresy detašovaných pracovísk: nie sú Vedúci detašovaných pracovísk: nie sú Typ organizácie: Rozpočtová od roku 1953 1.2. -

Entomopathogenic Fungi and Bacteria in a Veterinary Perspective
biology Review Entomopathogenic Fungi and Bacteria in a Veterinary Perspective Valentina Virginia Ebani 1,2,* and Francesca Mancianti 1,2 1 Department of Veterinary Sciences, University of Pisa, viale delle Piagge 2, 56124 Pisa, Italy; [email protected] 2 Interdepartmental Research Center “Nutraceuticals and Food for Health”, University of Pisa, via del Borghetto 80, 56124 Pisa, Italy * Correspondence: [email protected]; Tel.: +39-050-221-6968 Simple Summary: Several fungal species are well suited to control arthropods, being able to cause epizootic infection among them and most of them infect their host by direct penetration through the arthropod’s tegument. Most of organisms are related to the biological control of crop pests, but, more recently, have been applied to combat some livestock ectoparasites. Among the entomopathogenic bacteria, Bacillus thuringiensis, innocuous for humans, animals, and plants and isolated from different environments, showed the most relevant activity against arthropods. Its entomopathogenic property is related to the production of highly biodegradable proteins. Entomopathogenic fungi and bacteria are usually employed against agricultural pests, and some studies have focused on their use to control animal arthropods. However, risks of infections in animals and humans are possible; thus, further studies about their activity are necessary. Abstract: The present study aimed to review the papers dealing with the biological activity of fungi and bacteria against some mites and ticks of veterinary interest. In particular, the attention was turned to the research regarding acarid species, Dermanyssus gallinae and Psoroptes sp., which are the cause of severe threat in farm animals and, regarding ticks, also pets. -

Rickettsia Helvetica in Dermacentor Reticulatus Ticks
DISPATCHES The Study Rickettsia helvetica Using the cloth-dragging method, during March–May 2007 we collected 100 adult Dermacentor spp. ticks from in Dermacentor meadows in 2 different locations near Cakovec, between the Drava and Mura rivers in the central part of Medjimurje Coun- reticulatus Ticks ty. This area is situated in the northwestern part of Croatia, at Marinko Dobec, Dragutin Golubic, 46″38′N, 16″43′E, and has a continental climate with an Volga Punda-Polic, Franz Kaeppeli, average annual air temperature of 10.4°C at an altitude of and Martin Sievers 164 m. To isolate DNA from ticks, we modifi ed the method We report on the molecular evidence that Dermacentor used by Nilsson et al. (11). Before DNA isolation, ticks reticulatus ticks in Croatia are infected with Rickettsia hel- were disinfected in 70% ethanol and dried. Each tick was vetica (10%) or Rickettsia slovaca (2%) or co-infected with mechanically crushed in a Dispomix 25 tube with lysis buf- both species (1%). These fi ndings expand the knowledge of fer by using the Dispomix (Medic Tools, Zug, Switzerland). the geographic distribution of R. helvetica and D. reticulatus Lysis of each of the crushed tick samples was carried out in ticks. a solution of 6.7% sucrose, 0.2% proteinase K, 20 mg/mL lysozyme, and 10 ng/ml RNase A for 16 h at 37°C; 0.5 mo- ickettsia helvetica organisms were fi rst isolated from lar EDTA, and 20% sodium dodecyl sulfate was added and RIxodes ricinus ticks in Switzerland and were consid- further incubated for 1 h at 37°C. -

Central-European Ticks (Ixodoidea) - Key for Determination 61-92 ©Landesmuseum Joanneum Graz, Austria, Download Unter
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Mitteilungen der Abteilung für Zoologie am Landesmuseum Joanneum Graz Jahr/Year: 1972 Band/Volume: 01_1972 Autor(en)/Author(s): Nosek Josef, Sixl Wolf Artikel/Article: Central-European Ticks (Ixodoidea) - Key for determination 61-92 ©Landesmuseum Joanneum Graz, Austria, download unter www.biologiezentrum.at Mitt. Abt. Zool. Landesmus. Joanneum Jg. 1, H. 2 S. 61—92 Graz 1972 Central-European Ticks (Ixodoidea) — Key for determination — By J. NOSEK & W. SIXL in collaboration with P. KVICALA & H. WALTINGER With 18 plates Received September 3th 1972 61 (217) ©Landesmuseum Joanneum Graz, Austria, download unter www.biologiezentrum.at Dr. Josef NOSEK and Pavol KVICALA: Institute of Virology, Slovak Academy of Sciences, WHO-Reference- Center, Bratislava — CSSR. (Director: Univ.-Prof. Dr. D. BLASCOVIC.) Dr. Wolf SIXL: Institute of Hygiene, University of Graz, Austria. (Director: Univ.-Prof. Dr. J. R. MOSE.) Ing. Hanns WALTINGER: Centrum of Electron-Microscopy, Graz, Austria. (Director: Wirkl. Hofrat Dipl.-Ing. Dr. F. GRASENIK.) This study was supported by the „Jubiläumsfonds der österreichischen Nationalbank" (project-no: 404 and 632). For the authors: Dr. Wolf SIXL, Universität Graz, Hygiene-Institut, Univer- sitätsplatz 4, A-8010 Graz. 62 (218) ©Landesmuseum Joanneum Graz, Austria, download unter www.biologiezentrum.at Dedicated to ERICH REISINGER em. ord. Professor of Zoology of the University of Graz and corr. member of the Austrian Academy of Sciences 3* 63 (219) ©Landesmuseum Joanneum Graz, Austria, download unter www.biologiezentrum.at Preface The world wide distributed ticks, parasites of man and domestic as well as wild animals, also vectors of many diseases, are of great economic and medical importance. -

Full-Text PDF (Accepted Author Manuscript)
Medlock, J. M., Hansford, K. M., Vaux, A. G. C., Cull, B., Abdullah, S., Pietzsch, M. E., Wall, R. L., Johnson, N., & Phipps, L. P. (2017). Distribution of the tick Dermacentor reticulatus in the United Kingdom. Medical and Veterinary Entomology, 31(3), 281-288. https://doi.org/10.1111/mve.12235 Peer reviewed version License (if available): Unspecified Link to published version (if available): 10.1111/mve.12235 Link to publication record in Explore Bristol Research PDF-document This is the accepted author manuscript (AAM). The final published version (version of record) is available online via Wiley at https://doi.org/10.1111/mve.12235 . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ Distribution of the tick Dermacentor reticulatus in the United Kingdom Medlock, J.M.1,2, Hansford K.M.1, Vaux, A.G.C.1, Cull, B.1, Abdullah, S.3, Pietzsch, M.E.1, Wall, R.3, Johnson, N.4 & Phipps L.P.4 1 Medical Entomology group, Emergency Response Department, Public Health England, Porton Down, Salisbury, Wiltshire SP4 OJG. UK 2 Health Protection Research Unit in Emerging Infections & Zoonoses, Porton Down, Salisbury, Wiltshire SP4 0JG. UK 3 Veterinary Parasitology & Ecology group, School of Biological Sciences, University of Bristol, 24 Tyndall Avenue, Bristol BS8 1TQ UK 4 Wildlife Zoonoses and Vector-Borne Diseases Research Group, Animal and Plant Health Agency, Woodham Lane, Addlestone, Surrey, KT15 3NB UK Abstract The recent implication of Dermacentor reticulatus (Acari: Ixodidae) in the transmission of canine babesiosis in the United Kingdom (UK) has highlighted the lack of published accurate data on their distribution in the UK. -

Appendix E: Alternatives Analysis Report Integrated Mosquito and Vector Management Program
Integrated Mosquito and Vector Management Program APPENDIX E ALTERNATIVES ANALYSIS REPORT Appendix E: Alternatives Analysis Report Integrated Mosquito and Vector Management Program Document Information Prepared by Napa County Mosquito Abatement District Project Name Integrated Mosquito and Vector Management Program Draft Programmatic Environmental Impact Report Date July 2014 Prepared by: Napa County Mosquito Abatement District 15 Melvin Road, American Canyon, CA 94503 USA www.napamosquito.org With assistance from Cardno ENTRIX 2300 Clayton Road, Suite 200, Concord, CA 94520 USA www.cardno.com July 2014 NCMAD Document Information i MVCAC DPEIR_APP E_NCMAD AltRpt_MAR2016_R2.docx Appendix E: Alternatives Analysis Report Integrated Mosquito and Vector Management Program This Page Intentionally Left Blank ii Document Information NCMAD July 2014 MVCAC DPEIR_APP E_NCMAD AltRpt_MAR2016_R2.docx Appendix E: Alternatives Analysis Report Integrated Mosquito and Vector Management Program Table of Contents Introduction ............................................................................................................................1-1 1 Program Background ....................................................................................................1-1 1.1 Program Location ............................................................................................................. 1-1 1.2 Program History ................................................................................................................ 1-1 2 Potential Tools...............................................................................................................2-1 -

Checklist of British and Irish Hymenoptera - Chalcidoidea and Mymarommatoidea
Biodiversity Data Journal 4: e8013 doi: 10.3897/BDJ.4.e8013 Taxonomic Paper Checklist of British and Irish Hymenoptera - Chalcidoidea and Mymarommatoidea Natalie Dale-Skey‡, Richard R. Askew§‡, John S. Noyes , Laurence Livermore‡, Gavin R. Broad | ‡ The Natural History Museum, London, United Kingdom § private address, France, France | The Natural History Museum, London, London, United Kingdom Corresponding author: Gavin R. Broad ([email protected]) Academic editor: Pavel Stoev Received: 02 Feb 2016 | Accepted: 05 May 2016 | Published: 06 Jun 2016 Citation: Dale-Skey N, Askew R, Noyes J, Livermore L, Broad G (2016) Checklist of British and Irish Hymenoptera - Chalcidoidea and Mymarommatoidea. Biodiversity Data Journal 4: e8013. doi: 10.3897/ BDJ.4.e8013 Abstract Background A revised checklist of the British and Irish Chalcidoidea and Mymarommatoidea substantially updates the previous comprehensive checklist, dating from 1978. Country level data (i.e. occurrence in England, Scotland, Wales, Ireland and the Isle of Man) is reported where known. New information A total of 1754 British and Irish Chalcidoidea species represents a 22% increase on the number of British species known in 1978. Keywords Chalcidoidea, Mymarommatoidea, fauna. © Dale-Skey N et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. 2 Dale-Skey N et al. Introduction This paper continues the series of checklists of the Hymenoptera of Britain and Ireland, starting with Broad and Livermore (2014a), Broad and Livermore (2014b) and Liston et al. -

Molecular Phylogenetic Relationships of North American Dermacentor Ticks Using Mitochondrial Gene Sequences
Georgia Southern University Digital Commons@Georgia Southern Electronic Theses and Dissertations Graduate Studies, Jack N. Averitt College of Spring 2014 Molecular Phylogenetic Relationships of North American Dermacentor Ticks Using Mitochondrial Gene Sequences Kayla L. Perry Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/etd Part of the Biodiversity Commons, Bioinformatics Commons, Biology Commons, and the Molecular Biology Commons Recommended Citation Perry, Kayla L., "Molecular Phylogenetic Relationships of North American Dermacentor Ticks Using Mitochondrial Gene Sequences" (2014). Electronic Theses and Dissertations. 1089. https://digitalcommons.georgiasouthern.edu/etd/1089 This thesis (open access) is brought to you for free and open access by the Graduate Studies, Jack N. Averitt College of at Digital Commons@Georgia Southern. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. 1 MOLECULAR PHYLOGENETIC RELATIONSHIPS OF NORTH AMERICAN DERMACENTOR TICKS USING MITOCHONDRIAL GENE SEQUENCES by KAYLA PERRY (Under the Direction of Quentin Fang and Dmitry Apanaskevich) ABSTRACT Dermacentor is a recently evolved genus of hard ticks (Family Ixodiae) that includes 36 known species worldwide. Despite the importance of Dermacentor species as vectors of human and animal disease, the systematics of the genus remain largely unresolved. This study focuses on phylogenetic relationships of the eight North American Nearctic Dermacentor species: D. albipictus, D. variabilis, D. occidentalis, D. halli, D. parumapertus, D. hunteri, and D. andersoni, and the recently re-established species D. kamshadalus, as well as two of the Neotropical Dermacentor species D. nitens and D. dissimilis (both formerly Anocentor). -

Occurrence of Francisella Spp. in Dermacentor Reticulatus and Ixodes
Ticks and Tick-borne Diseases 6 (2015) 253–257 Contents lists available at ScienceDirect Ticks and Tick-borne Diseases j ournal homepage: www.elsevier.com/locate/ttbdis Short communication Occurrence of Francisella spp. in Dermacentor reticulatus and Ixodes ricinus ticks collected in eastern Poland a,∗ a a a a,b Angelina Wójcik-Fatla , Violetta Zajac˛ , Anna Sawczyn , Ewa Cisak , Jacek Sroka , a Jacek Dutkiewicz a Department of Zoonoses, Institute of Rural Health, Lublin, Poland b Department of Parasitology and Invasive Diseases, National Veterinary Research Institute, Pulawy, Poland a r t i c l e i n f o a b s t r a c t Article history: A total of 530 questing Dermacentor reticulatus ticks and 861 questing Ixodes ricinus ticks were collected Received 28 June 2014 from Lublin province (eastern Poland) and examined for the presence of Francisella by PCR for 16S rRNA Received in revised form (rrs) and tul4 genes. Only one female D. reticulatus tick out of 530 examined (0.2%) was infected with 19 December 2014 Francisella tularensis subspecies holarctica, as determined by PCR of the rrs gene. None of 861 I. ricinus Accepted 21 January 2015 ticks were infected with F. tularensis. In contrast, the presence of Francisella-like endosymbionts (FLEs) Available online 31 January 2015 was detected in more than half of the D. reticulatus ticks (50.4%) and 0.8% of the I. ricinus ticks. The nucleotide sequences of the FLEs detected in D. reticulatus exhibited 100% homology with the nucleotide Keywords: sequence of the FLE strain FDrH detected in Hungary in D. -
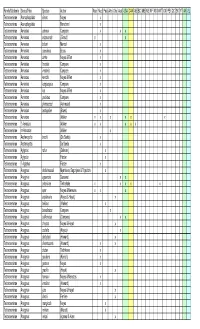
Encyrtidae Walker X X X X X X X X X X X X X X X X X X X X X
Family/Subfamily Genus/Tribe Species Author Near Neot Pala Afro Orie Aust USA CAN AB BC MB NB NF NS NWT ON PEI QC SK YT AK GL Tetracneminae Acerophagoides almon Noyes x Tetracneminae Acerophagoides Blanchard x Tetracneminae Aenasius advena Compere x x x Tetracneminae Aenasius arizonensis (Girault) x x Tetracneminae Aenasius bolowi Mercet x Tetracneminae Aenasius caeruleus Brues x Tetracneminae Aenasius cirrha Noyes & Ren x Tetracneminae Aenasius frontalis Compere x Tetracneminae Aenasius insularis Compere x Tetracneminae Aenasius kerrichi Noyes & Ren x Tetracneminae Aenasius longiscapus Compere x Tetracneminae Aenasius lua Noyes & Ren x Tetracneminae Aenasius paulistus Compere x Tetracneminae Aenasius phenacocci (Ashmead) x Tetracneminae Aenasius tachigaliae (Brues) x Tetracneminae Aenasius Walker x x x x x x Tetracneminae ? Aenasius Walker x x x x x Tetracneminae nr Aenasius Walker x Tetracneminae Aeptencyrtus bruchi (De Santis) x Tetracneminae Aeptencyrtus De Santis x Tetracneminae Aglyptus rufus (Dalman) x Tetracneminae Aglyptus Förster x Tetracneminae ? Aglyptus Förster x Tetracneminae Anagyrus abdulrassouli Myartseva, Sugonjaev & Trjapitzin x Tetracneminae Anagyrus agraensis Saraswat x x Tetracneminae Anagyrus antoninae Timberlake x x x x x Tetracneminae Anagyrus aper Noyes & Menezes x x x Tetracneminae Anagyrus aquilonaris (Noyes & Hayat) x Tetracneminae Anagyrus belibus (Walker) x Tetracneminae Anagyrus beneficians Compere x Tetracneminae Anagyrus californicus (Compere) x x Tetracneminae Anagyrus chrysos Noyes & Hayat x Tetracneminae -

Review Article Potential of Traditional Knowledge of Plants in the Management of Arthropods in Livestock Industry with Focus on (Acari) Ticks
Hindawi Evidence-Based Complementary and Alternative Medicine Volume 2017, Article ID 8647919, 33 pages https://doi.org/10.1155/2017/8647919 Review Article Potential of Traditional Knowledge of Plants in the Management of Arthropods in Livestock Industry with Focus on (Acari) Ticks Wycliffe Wanzala1,2 1 Department of Biological Sciences, School of Science and Information Sciences, Maasai Mara University, P.O.Box861-20500,Narok,Kenya 2Behavioural and Chemical Ecology Department, International Centre of Insect Physiology and Ecology, African Insect Science for Food and Health, P.O. Box 30772-00100-GPO, Nairobi, Kenya Correspondence should be addressed to Wycliffe Wanzala; [email protected] Received 19 December 2016; Accepted 11 May 2017; Published 17 July 2017 Academic Editor: Jose´ L. Rios Copyright © 2017 Wycliffe Wanzala. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Antitick plants and related ethnoknowledge/ethnopractices with potential for integrated tick control and management strategies to improve livestock production are reviewed. About 231 plants reviewed showed a variety of bioactive properties, namely, being toxic, repellent, antifeedant, and antiovipositant and ability to immobilize target tick species. These ethnobotanical substances are potentially useful in developing sustainable, efficient, and effective antitick agents suitable for rural livestock farmers.