Sceloporus Merriami Stejneger, 1904:17
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Herpetological Information Service No
Type Descriptions and Type Publications OF HoBART M. Smith, 1933 through June 1999 Ernest A. Liner Houma, Louisiana smithsonian herpetological information service no. 127 2000 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. Introduction Hobart M. Smith is one of herpetology's most prolific autiiors. As of 30 June 1999, he authored or co-authored 1367 publications covering a range of scholarly and popular papers dealing with such diverse subjects as taxonomy, life history, geographical distribution, checklists, nomenclatural problems, bibliographies, herpetological coins, anatomy, comparative anatomy textbooks, pet books, book reviews, abstracts, encyclopedia entries, prefaces and forwords as well as updating volumes being repnnted. The checklists of the herpetofauna of Mexico authored with Dr. Edward H. Taylor are legendary as is the Synopsis of the Herpetofalhva of Mexico coauthored with his late wife, Rozella B. -

Xenosaurus Tzacualtipantecus. the Zacualtipán Knob-Scaled Lizard Is Endemic to the Sierra Madre Oriental of Eastern Mexico
Xenosaurus tzacualtipantecus. The Zacualtipán knob-scaled lizard is endemic to the Sierra Madre Oriental of eastern Mexico. This medium-large lizard (female holotype measures 188 mm in total length) is known only from the vicinity of the type locality in eastern Hidalgo, at an elevation of 1,900 m in pine-oak forest, and a nearby locality at 2,000 m in northern Veracruz (Woolrich- Piña and Smith 2012). Xenosaurus tzacualtipantecus is thought to belong to the northern clade of the genus, which also contains X. newmanorum and X. platyceps (Bhullar 2011). As with its congeners, X. tzacualtipantecus is an inhabitant of crevices in limestone rocks. This species consumes beetles and lepidopteran larvae and gives birth to living young. The habitat of this lizard in the vicinity of the type locality is being deforested, and people in nearby towns have created an open garbage dump in this area. We determined its EVS as 17, in the middle of the high vulnerability category (see text for explanation), and its status by the IUCN and SEMAR- NAT presently are undetermined. This newly described endemic species is one of nine known species in the monogeneric family Xenosauridae, which is endemic to northern Mesoamerica (Mexico from Tamaulipas to Chiapas and into the montane portions of Alta Verapaz, Guatemala). All but one of these nine species is endemic to Mexico. Photo by Christian Berriozabal-Islas. amphibian-reptile-conservation.org 01 June 2013 | Volume 7 | Number 1 | e61 Copyright: © 2013 Wilson et al. This is an open-access article distributed under the terms of the Creative Com- mons Attribution–NonCommercial–NoDerivs 3.0 Unported License, which permits unrestricted use for non-com- Amphibian & Reptile Conservation 7(1): 1–47. -

)J Ieuican%Usellm
)J ox4tatesieuican%usellm PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK, N. Y. I0024 NUMBER 2450 FEBRUARY II, I971 Karyotypes of the Five Monotypic Species Groups of Lizards in the Genus Sceloporus BY CHARLES J. COLE INTRODUCTION Smith (1939) recognized 15 species groups in the large iguanid genus Sceloporus, which includes approximately 60 species. From one to 11 species are assigned to each group on the basis of relationships inferred from traits of external morphology. Five species of Sceloporus (some of them polytypic) are each sufficiently distinctive in external morphology to warrant assignment to monotypic species groups, implying that these five species appear to have no particularly close relatives extant. The present cytological investigation was undertaken to determine whether the karyo- types of these monotypic species groups are consistent with the data on external morphology, and whether the karyotypes suggest evolutionary relationships of these groups. The five species of Sceloporus that each comprise a monotypic group are: Sceloporus chrysostictus Cope, S. graciosus Baird and Girard, S. maculosus Smith, S. merriami Stejneger, and S. utiformis Cope. Each group is named for the species comprising it. My analysis of karyotypes from individuals of both sexes of each of these species forms the basis of the present report. METHODS Chromosome preparations were made by means of the colchicine, hypo- 1 Assistant Curator, Department of Herpetology, the American Museum of Natural History. 2 AMERICAN MUSEUM NOVITATES NO. 2450 tonic citrate technique used by Patton (1967), slightly modified for liz- ards (Lowe, Wright, and Cole, 1966). I used bone marrow and testicular tissues, and examined chromosomes in approximately 340 dividing cells from 28 lizards (19 males, 9 females; see Specimens Examined). -

REPTILIA: SQUAMATA: PHRYNOSOMATIDAE Sceloporus Poinsettii
856.1 REPTILIA: SQUAMATA: PHRYNOSOMATIDAE Sceloporus poinsettii Catalogue of American Amphibians and Reptiles. Webb, R.G. 2008. Sceloporus poinsettii. Sceloporus poinsettii Baird and Girard Crevice Spiny Lizard Sceloporus poinsettii Baird and Girard 1852:126. Type-locality, “Rio San Pedro of the Rio Grande del Norte, and the province of Sonora,” restricted to either the southern part of the Big Burro Moun- tains or the vicinity of Santa Rita, Grant County, New Mexico by Webb (1988). Lectotype, National Figure 1. Adult male Sceloporus poinsettii poinsettii (UTEP Museum of Natural History (USNM) 2952 (subse- 8714) from the Magdalena Mountains, Socorro County, quently recataloged as USNM 292580), adult New Mexico (photo by author). male, collected by John H. Clark in company with Col. James D. Graham during his tenure with the U.S.-Mexican Boundary Commission in late Au- gust 1851 (examined by author). See Remarks. Sceloporus poinsetii: Duméril 1858:547. Lapsus. Tropidolepis poinsetti: Dugès 1869:143. Invalid emendation (see Remarks). Sceloporus torquatus Var. C.: Bocourt 1874:173. Sceloporus poinsetti: Yarrow “1882"[1883]:58. Invalid emendation. S.[celoporus] t.[orquatus] poinsettii: Cope 1885:402. Seloporus poinsettiii: Herrick, Terry, and Herrick 1899:123. Lapsus. Sceloporus torquatus poinsetti: Brown 1903:546. Sceloporus poissetti: Král 1969:187. Lapsus. Figure 2. Adult female Sceloporus poinsettii axtelli (UTEP S.[celoporus] poinssetti: Méndez-De la Cruz and Gu- 11510) from Alamo Mountain (Cornudas Mountains), tiérrez-Mayén 1991:2. Lapsus. Otero County, New Mexico (photo by author). Scelophorus poinsettii: Cloud, Mallouf, Mercado-Al- linger, Hoyt, Kenmotsu, Sanchez, and Madrid 1994:119. Lapsus. Sceloporus poinsetti aureolus: Auth, Smith, Brown, and Lintz 2000:72. -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

The Evolution of Demographic Tactics in Lizards: a Test of Some Hypotheses Concerning Life History Evolution
J. evol. biol. 11 (1998) 329–364 1010–061X/98/030329–36 $ 1.50+0.20/0 The evolution of demographic tactics in lizards: a test of some hypotheses concerning life history evolution J. Clobert,1 T. Garland Jr.2 and R. Barbault1 1Laboratoire d’Ecologie, Uni6ersite´ Pierre et Marie Curie, Baˆtiment A, Case 237, 7 quai Saint Bernard, 75252 Paris cedex 05, France 2Department of Zoology, 430 Lincoln Dri6e, Uni6ersity of Wisconsin, Madison, WI 53706-1381, USA Key words: Comparative methods; demographic tactics; life history; phylogeny; dimension numbers; lizards. Abstract We analyze, with an augmented data base, patterns of covariation of the three primary demographic parameters (age at maturity, fecundity, adult survival, all measured in the same unit of time) in lizards. This also constitutes a first attempt to use all three of these parameters for this group of species. We attempt to place these analyses in the framework of recent theories on life history evolution (Ferrie`re and Clobert, 1992; Charnov, 1993). Life history data were collected from the literature and from our original work, and a composite phylogeny was assembled, based on a variety of published sources. Using a phylogenetically based statistical method (independent contrasts), the allometric (log-log) relationship of fecundity (and of clutch size) in relation to snout-vent length was found to differ significantly between the two major clades of extant lizards, Iguania (43 species in our data set) and Scleroglossa (47 species). We therefore emphasize analyses done separately for the two clades. Without removing correlations with body size, the relationships between fecundity and survival, and between fecundity and age at maturity, were also found to differ between clades, which differs from Charnov’s (1993) predic- tions. -

Amphibians and Reptiles of the State of Coahuila, Mexico, with Comparison with Adjoining States
A peer-reviewed open-access journal ZooKeys 593: 117–137Amphibians (2016) and reptiles of the state of Coahuila, Mexico, with comparison... 117 doi: 10.3897/zookeys.593.8484 CHECKLIST http://zookeys.pensoft.net Launched to accelerate biodiversity research Amphibians and reptiles of the state of Coahuila, Mexico, with comparison with adjoining states Julio A. Lemos-Espinal1, Geoffrey R. Smith2 1 Laboratorio de Ecología-UBIPRO, FES Iztacala UNAM. Avenida los Barrios 1, Los Reyes Iztacala, Tlalnepantla, edo. de México, Mexico – 54090 2 Department of Biology, Denison University, Granville, OH, USA 43023 Corresponding author: Julio A. Lemos-Espinal ([email protected]) Academic editor: A. Herrel | Received 15 March 2016 | Accepted 25 April 2016 | Published 26 May 2016 http://zoobank.org/F70B9F37-0742-486F-9B87-F9E64F993E1E Citation: Lemos-Espinal JA, Smith GR (2016) Amphibians and reptiles of the state of Coahuila, Mexico, with comparison with adjoining statese. ZooKeys 593: 117–137. doi: 10.3897/zookeys.593.8484 Abstract We compiled a checklist of the amphibians and reptiles of the state of Coahuila, Mexico. The list com- prises 133 species (24 amphibians, 109 reptiles), representing 27 families (9 amphibians, 18 reptiles) and 65 genera (16 amphibians, 49 reptiles). Coahuila has a high richness of lizards in the genus Sceloporus. Coahuila has relatively few state endemics, but has several regional endemics. Overlap in the herpetofauna of Coahuila and bordering states is fairly extensive. Of the 132 species of native amphibians and reptiles, eight are listed as Vulnerable, six as Near Threatened, and six as Endangered in the IUCN Red List. In the SEMARNAT listing, 19 species are Subject to Special Protection, 26 are Threatened, and three are in Danger of Extinction. -

Collection, Trade, and Regulation of Reptiles and Amphibians of the Chihuahuan Desert Ecoregion
Collection, Trade, and Regulation of Reptiles and Amphibians of the Chihuahuan Desert Ecoregion Lee A. Fitzgerald, Charles W. Painter, Adrian Reuter, and Craig Hoover COLLECTION, TRADE, AND REGULATION OF REPTILES AND AMPHIBIANS OF THE CHIHUAHUAN DESERT ECOREGION By Lee A. Fitzgerald, Charles W. Painter, Adrian Reuter, and Craig Hoover August 2004 TRAFFIC North America World Wildlife Fund 1250 24th Street NW Washington DC 20037 Visit www.traffic.org for an electronic edition of this report, and for more information about TRAFFIC North America. © 2004 WWF. All rights reserved by World Wildlife Fund, Inc. ISBN 0-89164-170-X All material appearing in this publication is copyrighted and may be reproduced with permission. Any reproduction, in full or in part, of this publication must credit TRAFFIC North America. The views of the authors expressed in this publication do not necessarily reflect those of the TRAFFIC Network, World Wildlife Fund (WWF), or IUCN-The World Conservation Union. The designation of geographical entities in this publication and the presentation of the material do not imply the expression of any opinion whatsoever on the part of TRAFFIC or its supporting organizations concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The TRAFFIC symbol copyright and Registered Trademark ownership are held by WWF. TRAFFIC is a joint program of WWF and IUCN. Suggested citation: Fitzgerald, L.A., et al. 2004. Collection, Trade, and Regulation of Reptiles and Amphibians of the Chihuahuan Desert Ecoregion. TRAFFIC North America. Washington D.C.: World Wildlife Fund. -

Phylogenetic Relationships of Phrynosomatid Lizards Based on Nuclear and Mitochondrial Data, and a Revised Phylogeny for Sceloporus
Molecular Phylogenetics and Evolution 54 (2010) 150–161 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Phylogenetic relationships of phrynosomatid lizards based on nuclear and mitochondrial data, and a revised phylogeny for Sceloporus John J. Wiens a,*, Caitlin A. Kuczynski a, Saad Arif a, Tod W. Reeder b a Department of Ecology and Evolution, Stony Brook University, Stony Brook, NY 11794-5245, USA b Department of Biology, San Diego State University, San Diego, CA 92182-4164, USA article info abstract Article history: Phrynosomatid lizards are among the most common and diverse groups of reptiles in western North Received 31 March 2009 America, Mexico, and Central America. Phrynosomatidae includes 136 species in 10 genera. Phrynoso- Revised 10 August 2009 matids are used as model systems in many research programs in evolution and ecology, and much of this Accepted 8 September 2009 research has been undertaken in a comparative phylogenetic framework. However, relationships among Available online 12 September 2009 many phrynosomatid genera are poorly supported and in conflict between recent studies. Further, pre- vious studies based on mitochondrial DNA sequences suggested that the most species-rich genus (Scelop- Keywords: orus) is possibly paraphyletic with respect to as many as four other genera (Petrosaurus, Sator, Urosaurus, Mitochondrial DNA and Uta). Here, we collect new sequence data from five nuclear genes and combine them with published Nuclear DNA Phrynosomatidae data from one additional nuclear gene and five mitochondrial gene regions. We compare trees from Phylogeny nuclear and mitochondrial data from 37 phrynosomatid taxa, including a ‘‘species tree” (from BEST) Reptiles for the nuclear data. -

Reproductive Ecology of Sceloporus Utiformis (Sauria: Phrynosomatidae) from a Tropical Dry Forest of Me´Xico
Journal of Herpetology, Vol. 37, No. 1, pp. 1±10, 2003 Copyright 2003 Society for the Study of Amphibians and Reptiles Reproductive Ecology of Sceloporus utiformis (Sauria: Phrynosomatidae) from a Tropical Dry Forest of MeÂxico AURELIO RAMIÂREZ-BAUTISTA1,2,3 AND GUADALUPE GUTIEÂ RREZ-MAYEÂ N4 1Laboratorio de EcologõÂa, Unidad de BiologõÂa, TecnologõÂa y Prototipos (UBIPRO), FES-Iztacala, Universidad Nacional AutoÂnoma de MeÂxico. Av. de los Barrios s/n, Los Reyes Iztacala, Tlalnepantla, Edo. de Mex. C.P. 54090, A.P. 314. MeÂxico; E-mail: [email protected] 4Laboratorio de HerpetologõÂa. Escuela de BiologõÂa. BenemeÂrita Universidad AutoÂnoma de Puebla. Edi®cio 76. Ciudad Universitaria. Av. San Claudio y Blvd. Valsequillo s/n. Col. San Manuel. C.P. 72570. Puebla, Pue. MeÂxico; E-mail: [email protected] ABSTRACT.ÐThe reproductive cycle and cycles in fat body and liver mass are described for male and female Sceloporus utiformis from Chamela, Jalisco, MeÂxico, during 1988 and 1990. These cycles varied among months and between sexes. Males reached sexual maturity at 45 mm snout±vent length (SVL) at an age of seven months. Females reached sexual maturity at 56 mm at an age of nine months. Testes of males began to increase in volume in early May, and maximal testicular activity occurred from June to October. Testis size began to decrease in November and was small in December. Reproductive activity of females occurred between July and March. Gonads of females began to increase in volume in mid-June, and maximal egg production was from August to November. Maximal testicular growth was positively correlated with in- creasing temperature and precipitation, but not with photoperiod, whereas in females follicular growth was positively correlated with photoperiod but not with temperature and precipitation. -

ZOO 4462C – Herpetology Spring 2021, 4 Credits
ZOO 4462C – Herpetology Spring 2021, 4 credits Course Schedule – See page 10 Instructor: Dr. Gregg Klowden (pronounced "Cloud - in”) Office: Room 202A, Biological Sciences E-mail: [email protected] Phone: Please send an email instead Mark Catesby (1731) “Natural History of Carolina, Florida and the Bahama Islands” "These foul and loathsome animals . are abhorrent because of their cold body, pale color, cartilaginous skeleton, filthy skin, fierce aspect, calculating eye, offensive smell, harsh voice, squalid habitation, and terrible venom; and so their Creator has not exerted his powers to make many of them." Carolus Linnaeus (1758) ***Email Requirements: I teach several courses and receive a large volume of emails. To help me help you please: 1. format the subject of your email as follows: “Course – Herpetology, Subject - Question about exam 1” 2. include your 1st and last name in the body of all correspondence. I try to respond to emails within 48 hours however, response time may be greater. o Please plan accordingly by not waiting to the last minute to contact me with questions or concerns. All messaging must be done using either Webcourses or your Knight's E-Mail. o Messages from non-UCF addresses will not be answered. Due to confidentiality, questions about grades should be sent via Webcourses messaging, not via email. Office Hours: Tuesdays and Thursdays 10:30-11:30a and 2:00-3:00p or by appointment All office hours will be held online via Zoom. An appointment is not necessary. Just log into Zoom using the link posted on Webcourses. You will initially be admitted to a waiting room and Dr. -
2016 JMIH Program Book Onsite Changes.Pub
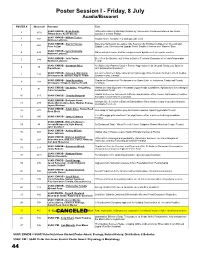
Poster Session I - Friday, 8 July Acadia/Bissonet POSTER # Abstract # Presenter Title SSAR: ENHDB - Brian Devlin, Differential Rates of Malarial Infection by Plasmodium floridense between two Anolis 1 1010 Tiffany Doan, Kevin Greene species in Central Florida SSAR: ENHDB - William Ternes, 2 392 Trophic Niche Variation in a Widespread Lizard Matthew Lattanzio SSAR: ENHDB - Alex A Thomas, Experimental Test of Overwinter Site Selection by Ectotherms Based on Thermal and 3 265 Peter A Zani Spatial Cues: Side-blotched Lizards Prefer Smaller Crevices over Warmer Sites SSAR: ENHDB - Kylie Krohmaly, 4 432 Male mate preference and the complex social dynamics of Urosaurus ornatus Matthew Lattanzio SSAR: ENHDB - Julie Taylor, Blue Gets the Boulder, but Yellow is Bolder: Territorial Dynamics of a Color Polymorphic 5 388 Matthew Lattanzio Lizard SSAR: ENHDB - Savannah Price, Are Blue Color Patches Used in Female Aggression in an Unusual Sceloporus Species 6 46 Diana Hews with Ornamented Females? SSAR: ENHDB - Kelsey A. Marchand, Life on the Northern Edge: Overwintering Ecology of the Western Painted Turtle in Regina, 7 134 Christopher M. Somers, Ray G. Poulin Saskatchewan, Canada SSAR: ENHDB - John Konvalina, Population Dynamics of Chelonians in an Urban Lake in Jonesboro, Craighead County, 8 181 Christopher Thigpen, Stanley Trauth Arkansas SSAR: ENHDB - Iwo Gross, Yong Wang, Habitat use and dispersal of neonatal Copperheads (Crotalinae; Agkistrodon) in a managed 9 91 Callie Schweitzer southeastern forest Habitat Preference, Movement Patterns, and