CDC Fact Sheet Ebola Virus Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development, Manufacturing, and Supply of MSD's Ebola Vaccine
Engineering Conferences International ECI Digital Archives Vaccine Technology VI Proceedings 6-15-2016 Development, manufacturing, and supply of MSD’s Ebola vaccine Jeffrey T. Blue Director Vaccine Drug Product Development, Merck Sharp & Dohme Corp., USA, [email protected] Follow this and additional works at: http://dc.engconfintl.org/vaccine_vi Part of the Engineering Commons Recommended Citation Jeffrey T. Blue, "Development, manufacturing, and supply of MSD’s Ebola vaccine" in "Vaccine Technology VI", Laura Palomares, UNAM, Mexico Manon Cox, Protein Sciences Corporation, USA Tarit Mukhopadhyay, University College London, UK Nathalie Garçon, BIOASTER Technology Research Institute, FR Eds, ECI Symposium Series, (2016). http://dc.engconfintl.org/vaccine_vi/ 23 This Abstract and Presentation is brought to you for free and open access by the Proceedings at ECI Digital Archives. It has been accepted for inclusion in Vaccine Technology VI by an authorized administrator of ECI Digital Archives. For more information, please contact [email protected]. Development, Manufacturing, and Supply of MSD’s Ebola Vaccine Jeffrey T. Blue Director, Vaccine Drug Product Development Merck & Co. Inc. Vaccine Technology VI 15 June 2016 Albufeira, Portugal • Elements of this program has been funded in whole or in part with Federal Funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority under Contract Number: HHSO100201500002C Presentation -

COVID-19: Perspective, Patterns and Evolving Strategies
COVID-19: Perspective, Patterns and Evolving strategies Subject Category: Clinical Virology Vinod Nikhra* Department of Medicine, Hindu Rao Hospital & NDMC Medical College, New Delhi, India Submitted: 02 June 2020 | Approved: 06 July 2020 | Published: 09 July 2020 Copyright: © 2020 Nikhra V. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. DOI: https://dx.doi.org/10.29328/ebook1003 ORCID: https://orcid.org/0000-0003-0859-5232 *Corresponding author: Dr. Vinod Nikhra, M.D. Consultant and Faculty, Department of Medicine, Hindu Rao Hospital & NDMC Medical College, New Delhi, India, Tel: 91-9810874937; Email: [email protected]; drvinodnikhra@rediff mail.com Open Access COVID-19: Perspective, Patterns and Evolving strategies Table of Contents - 7 Chapters Sl No Chapters Title Pages The Trans-Zoonotic Virome Interface: Measures to 1 Chapter 1 003-011 Balance, Control and Treat Epidemics Exploring Pathophysiology of COVID-19 Infection: Faux 2 Chapter 2 012-020 Espoir and Dormant Therapeutic Options The Agent and Host Factors in COVID-19: Exploring 3 Chapter 3 021-036 Pathogenesis and Therapeutic Implications Adverse Outcomes for Elderly in COVID-19: Annihilation 4 Chapter 4 037-047 of the Longevity Dream Identifying Patterns in COVID-19: Morbidity, Recovery, 5 Chapter 5 048-058 and the Aftermath The New Revelations: Little-known Facts about COVID-19 6 Chapter 6 059-068 and their Implications Fear, Reaction and Rational Behaviour to COVID-19 in 7 Chapter 7 069-076 Public, Health Professionals and Policy Planners La Confusion: Caring for COVID-19 patients 8 Postscript 077-079 and the raging, engulfi ng and debilitating pandemic 9 Acknowledgement 080-080 *Corresponding HTTPS://WWW.HEIGHPUBS.ORG author: Dr. -

Signature Redacted Signature of Author
Developing VHH-based tools to study Ebolavirus Infection By Jason V. M. H. Nguyen B.S., Biochemistry and Molecular Biology University of California, Santa Cruz, 2012 Submitted to the Microbiology Graduate Program in partial fulfillment of the requirements for the degree of Doctor of Philosophy At the Massachusetts Institute of Technology June 2019 2019 Massachusetts Institute of Technology. All rights reserved. Signature redacted Signature of Author...... .................................................................... V Y' Jason V. M. H. Nguyen Microbiology Graduate Program May 13, 2019 Signature redacted Certified by.............. ................................................................... Hidde L. Ploegh Senior Investigator Boston Children's Hospital It Signature redacted Accepted by....... ......................................................................... Jacquin C. Niles Associate Professor of Biological Engineering Chair of Microbiology Program MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUN 2 12019 1 LIBRARIES ARCHIVES 2 Developing VHH-based tools to study Ebolavirus Infection By Jason V. M. H. Nguyen Submitted to the Microbiology Graduate Program on May 13th, 2019 in partial fulfillment of the requirements for the degree of Doctor of Philosophy ABSTRACT Variable domains of camelid-derived heavy chain-only antibodies, or VHHs, have emerged as a unique antigen binding moiety that holds promise in its versatility and utilization as a tool to study biological questions. This thesis focuses on two aspects on developing tools to study infectious disease, specifically Ebolavirus entry. In Chapter 1, I provide an overview about antibodies and how antibodies have transformed the biomedical field and how single domain antibody fragments, or VHHs, have entered this arena. I will also touch upon how VHHs have been used in various fields and certain aspects that remain underexplored. Chapter 2 focuses on the utilization of VHHs to study Ebolavirus entry using VHHs that were isolated from alpacas. -

Assembling Evidence for Identifying Reservoirs of Infection
TREE-1806; No. of Pages 10 Review Assembling evidence for identifying reservoirs of infection 1 1,2 1,3 1 4 Mafalda Viana , Rebecca Mancy , Roman Biek , Sarah Cleaveland , Paul C. Cross , 3,5 1 James O. Lloyd-Smith , and Daniel T. Haydon 1 Boyd Orr Centre for Population and Ecosystem Health, Institute of Biodiversity, Animal Health and Comparative Medicine, College of Medical Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, UK 2 School of Computing Science, University of Glasgow, Glasgow G12 8QQ, UK 3 Fogarty International Center, National Institutes of Health, Bethesda, MD 20892, USA 4 US Geological Survey, Northern Rocky Mountain Science Center 2327, University Way, Suite 2, Bozeman, MT 59715, USA 5 Department of Ecology and Evolutionary Biology, University of California at Los Angeles, Los Angeles, CA 90095, USA Many pathogens persist in multihost systems, making patterns of incidence and prevalence (see Glossary) that the identification of infection reservoirs crucial for result from the connectivity between source and target devising effective interventions. Here, we present a con- populations (black arrows in Figure I in Box 1). We then ceptual framework for classifying patterns of incidence review methods that allow us to identify maintenance or and prevalence, and review recent scientific advances nonmaintenance populations (squares or circles in Figure that allow us to study and manage reservoirs simulta- I, Box 1), how they are connected (arrows in Figure I, Box neously. We argue that interventions can have a crucial 1), and the role that each of these populations has in role in enriching our mechanistic understanding of how maintaining the pathogen (i.e., reservoir capacity). -

Democratic Republic of the Congo – Ebola Outbreaks SEPTEMBER 30, 2020
Fact Sheet #10 Fiscal Year (FY) 2020 Democratic Republic of the Congo – Ebola Outbreaks SEPTEMBER 30, 2020 SITUATION AT A GLANCE 128 53 13 3,470 2,287 Total Confirmed and Total EVD-Related Total EVD-Affected Total Confirmed and Total EVD-Related Probable EVD Cases in Deaths in Équateur Health Zones in Probable EVD Cases in Deaths in Eastern DRC Équateur Équateur Eastern DRC at End of at End of Outbreak Outbreak MoH – September 30, 2020 MoH – September 30, 2020 MoH – September 30, 2020 MoH – June 25, 2020 MoH – June 25, 2020 Health actors remain concerned about surveillance gaps in northwestern DRC’s Équateur Province. In recent weeks, several contacts of EVD patients have travelled undetected to neighboring RoC and the DRC’s Mai- Ndombe Province, heightening the risk of regional EVD spread. Logistics coordination in Equateur has significantly improved in recent weeks, with response actors establishing a Logistics Cluster in September. The 90-day enhanced surveillance period in eastern DRC ended on September 25. TOTAL USAID HUMANITARIAN FUNDING USAID/BHA1,2 $152,614,242 For the DRC Ebola Outbreaks Response in FY 2020 USAID/GH in $2,500,000 Neighboring Countries3 For complete funding breakdown with partners, see funding chart on page 6 Total $155,114,2424 1USAID’s Bureau for Humanitarian Assistance (USAID/BHA) 2 Total USAID/BHA funding includes non-food humanitarian assistance from the former Office of U.S. Foreign Disaster Assistance. 3 USAID’s Bureau for Global Health (USAID/GH) 4 Some of the USAID funding intended for Ebola virus disease (EVD)-related programs in eastern Democratic Republic of the Congo (DRC) is now supporting EVD response activities in Équateur. -

Variation in Antimicrobial Susceptibility Among Borrelia Burgdorferi Strains? Emir Hodzic*
BOSNIAN JOURNAL OF BASIC MEDICAL SCIENCES REVIEW WWW.BJBMS.ORG Lyme Borreliosis: is there a preexisting (natural) variation in antimicrobial susceptibility among Borrelia burgdorferi strains? Emir Hodzic* Real-Time PCR Research and Diagnostic Core Facility, School of Veterinary Medicine, University of California at Davis, California, United States of America ABSTRACT The development of antibiotics changed the world of medicine and has saved countless human and animal lives. Bacterial resistance/tolerance to antibiotics have spread silently across the world and has emerged as a major public health concern. The recent emergence of pan-resistant bacteria can overcome virtually any antibiotic and poses a major problem for their successful control. Selection for antibiotic resistance may take place where an antibiotic is present: in the skin, gut, and other tissues of humans and animals and in the environment. Borrelia burgdorferi, the etiological agents of Lyme borreliosis, evades host immunity and establishes persistent infections in its mammalian hosts. The persistent infection poses a challenge to the effective antibiotic treatment, as demonstrated in various animal models. An increasingly heterogeneous sub- population of replicatively attenuated spirochetes arises following treatment, and these persistent antimicrobial tolerant/resistant spirochetes are non-cultivable. The non-cultivable spirochetes resurge in multiple tissues at 12 months after treatment, withB. burgdorferi-specific DNA copy levels nearly equivalent to those found in shame-treated experimental animals. These attenuated spirochetes remain viable, but divide slowly, thereby being tolerant to antibiotics. Despite the continued non-cultivable state, RNA transcription of multiple B. burgdorferi genes was detected in host tissues, spirochetes were acquired by xenodiagnostic ticks, and spirochetal forms could be visualized within ticks and mouse tissues. -

Dengue Fever in Senegal 6 - 7 Ongoing Events Ebola Virus Disease in the Democratic Republic of the Congo Humanitarian Crisis in Cameroon
Overview Contents This Weekly Bulletin focuses on selected acute public health emergencies occurring in the WHO African Region. The WHO Health Emergencies Programme is currently monitoring 58 events in the region. This week’s edition covers key new and ongoing events, including: 2 Overview Hepatitis E in Central African Republic 3 - 5 New events Monkeypox in Central African Republic Dengue fever in Senegal 6 - 7 Ongoing events Ebola virus disease in the Democratic Republic of the Congo Humanitarian crisis in Cameroon. 8 Summary of major issues challenges and For each of these events, a brief description, followed by public health proposed actions measures implemented and an interpretation of the situation is provided. 9 All events currently A table is provided at the end of the bulletin with information on all new and being monitored ongoing public health events currently being monitored in the region, as well as events that have recently been closed. Major issues and challenges include: The Ebola virus disease (EVD) outbreak in the Democratic Republic of the Congo has reached a critical juncture, marked by a precarious security situation, persistence of pockets of community resistance/ mistrust and expanding geographical spread of the disease. During the reporting week, there was an incident involving a response team performing burial activity in Butembo. This came barely days following a widespread community strike (“ville morte”) in Beni and several towns, and an earlier armed attack in Beni. These incidents severely disrupted most outbreak control interventions. Meanwhile, EVD cases have been confirmed in new areas with worse insecurity and in close proximity to the border with Uganda. -

Impact of Babesia Microti Infection on the Initiation and Course Of
Tołkacz et al. Parasites Vectors (2021) 14:132 https://doi.org/10.1186/s13071-021-04638-0 Parasites & Vectors RESEARCH Open Access Impact of Babesia microti infection on the initiation and course of pregnancy in BALB/c mice Katarzyna Tołkacz1,2*, Anna Rodo3, Agnieszka Wdowiarska4, Anna Bajer1† and Małgorzata Bednarska5† Abstract Background: Protozoa in the genus Babesia are transmitted to humans through tick bites and cause babesiosis, a malaria-like illness. Vertical transmission of Babesia spp. has been reported in mammals; however, the exact timing and mechanisms involved are not currently known. The aims of this study were to evaluate the success of vertical transmission of B. microti in female mice infected before pregnancy (mated during the acute or chronic phases of Babesia infection) and that of pregnant mice infected during early and advanced pregnancy; to evaluate the pos- sible infuence of pregnancy on the course of parasite infections (parasitaemia); and to assess pathological changes induced by parasitic infection. Methods: The frst set of experiments involved two groups of female mice infected with B. microti before mating, and inseminated on the 7th day and after the 40th day post infection. A second set of experiments involved female mice infected with B. microti during pregnancy, on the 4th and 12th days of pregnancy. Blood smears and PCR targeting the 559 bp 18S rRNA gene fragment were used for the detection of B. microti. Pathology was assessed histologically. Results: Successful development of pregnancy was recorded only in females mated during the chronic phase of infection. The success of vertical transmission of B. -

2019 Icar Program & Abstracts Book
Hosted by the International Society for Antiviral Research (ISAR) ND International Conference 32on Antiviral Research (ICAR) Baltimore MARYLAND PROGRAM and USA Hyatt Regency BALTIMORE ABSTRACTS May 12-15 2019 ND TABLE OF International Conference CONTENTS 32on Antiviral Research (ICAR) Daily Schedule . .3 Organization . 4 Contributors . 5 Keynotes & Networking . 6 Schedule at a Glance . 7 ISAR Awardees . 10 The 2019 Chu Family Foundation Scholarship Awardees . 15 Speaker Biographies . 17 Program Schedule . .25 Posters . 37 Abstracts . 53 Author Index . 130 PROGRAM and ABSTRACTS of the 32nd International Conference on Antiviral Research (ICAR) 2 ND DAILY International Conference SCHEDULE 32on Antiviral Research (ICAR) SUNDAY, MAY 12, 2019 › Women in Science Roundtable › Welcome and Keynote Lectures › Antonín Holý Memorial Award Lecture › Influenza Symposium › Opening Reception MONDAY, MAY 13, 2019 › Women in Science Award Lecture › Emerging Virus Symposium › Short Presentations 1 › Poster Session 1 › Retrovirus Symposium › ISAR Award of Excellence Presentation › PechaKucha Event with Introduction of First Time Attendees TUESDAY, MAY 14, 2019 › What’s New in Antiviral Research 1 › Short Presentations 2 & 3 › ISAR Award for Outstanding Contributions to the Society Presentation › Career Development Panel › William Prusoff Young Investigator Award Lecture › Medicinal Chemistry Symposium › Poster Session 2 › Networking Reception WEDNESDAY, MAY 15, 2019 › Gertrude Elion Memorial Award Lecture › What’s New in Antiviral Research 2 › Shotgun Oral -

To Ebola Reston
WHO/HSE/EPR/2009.2 WHO experts consultation on Ebola Reston pathogenicity in humans Geneva, Switzerland 1 April 2009 EPIDEMIC AND PANDEMIC ALERT AND RESPONSE WHO experts consultation on Ebola Reston pathogenicity in humans Geneva, Switzerland 1 April 2009 © World Health Organization 2009 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distin- guished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. This publication contains the collective views of an international group of experts and does not necessarily represent the decisions or the policies of the World Health Organization. -

How Severe and Prevalent Are Ebola and Marburg Viruses?
Nyakarahuka et al. BMC Infectious Diseases (2016) 16:708 DOI 10.1186/s12879-016-2045-6 RESEARCHARTICLE Open Access How severe and prevalent are Ebola and Marburg viruses? A systematic review and meta-analysis of the case fatality rates and seroprevalence Luke Nyakarahuka1,2,5* , Clovice Kankya2, Randi Krontveit3, Benjamin Mayer4, Frank N. Mwiine2, Julius Lutwama5 and Eystein Skjerve1 Abstract Background: Ebola and Marburg virus diseases are said to occur at a low prevalence, but are very severe diseases with high lethalities. The fatality rates reported in different outbreaks ranged from 24–100%. In addition, sero-surveys conducted have shown different seropositivity for both Ebola and Marburg viruses. We aimed to use a meta-analysis approach to estimate the case fatality and seroprevalence rates of these filoviruses, providing vital information for epidemic response and preparedness in countries affected by these diseases. Methods: Published literature was retrieved through a search of databases. Articles were included if they reported number of deaths, cases, and seropositivity. We further cross-referenced with ministries of health, WHO and CDC databases. The effect size was proportion represented by case fatality rate (CFR) and seroprevalence. Analysis was done using the metaprop command in STATA. Results: The weighted average CFR of Ebola virus disease was estimated to be 65.0% [95% CI (54.0–76.0%), I2 = 97.98%] whereas that of Marburg virus disease was 53.8% (26.5–80.0%, I2 = 88.6%). The overall seroprevalence of Ebola virus was 8.0% (5.0%–11.0%, I2 = 98.7%), whereas that for Marburg virus was 1.2% (0.5–2.0%, I2 = 94.8%). -
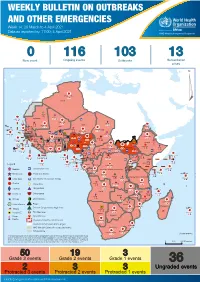
Weekly Bulletin on Outbreaks
WEEKLY BULLETIN ON OUTBREAKS AND OTHER EMERGENCIES Week 14: 29 March to 4 April 2021 Data as reported by: 17:00; 4 April 2021 REGIONAL OFFICE FOR Africa WHO Health Emergencies Programme 0 116 103 13 New event Ongoing events Outbreaks Humanitarian crises 117 622 3 105 Algeria ¤ 36 13 110 0 5 420 164 Mauritania 7 2 10 501 392 110 0 7 0 Niger 17 927 449 Mali 3 334 10 567 0 6 0 2 079 4 4 595 165 Eritrea Cape Verde 38 520 1 037 Chad Senegal 4 918 185 59 0 Gambia 27 0 3 0 17 125 159 9 761 45 Guinea-Bissau 796 17 7 0 Burkina Faso 225 46 215 189 2 963 0 162 593 2 048 Guinea 12 817 150 12 38 397 1 3 662 66 1 1 23 12 Benin 30 0 Nigeria 1 873 71 0 Ethiopia 420 14 481 5 6 188 15 Sierra Leone Togo 3 473 296 53 920 779 52 14 Ghana 5 245 72 Côte d'Ivoire 10 098 108 14 484 479 63 0 40 0 Liberia 17 0 South Sudan Central African Republic 916 2 45 0 25 0 19 670 120 43 180 237 90 287 740 Cameroon 7 0 28 676 137 5 330 13 138 988 2 224 1 952 87 655 2 51 22 43 0 112 12 6 1 488 6 3 988 79 11 187 6 902 102 Equatorial Guinea Uganda 542 8 Sao Tome and Principe 32 11 2 042 85 41 016 335 Kenya Legend 7 100 90 Gabon Congo 18 504 301 Rwanda Humanitarian crisis 2 212 34 22 482 311 Measles 18 777 111 Democratic Republic of the Congo 9 681 135 Burundi 2 964 6 Monkeypox Ebola virus disease Seychelles 27 930 739 1 525 0 420 29 United Republic of Tanzania Lassa fever Skin disease of unknown etiology 189 0 4 084 20 509 21 Cholera Yellow fever 1 349 5 6 257 229 22 631 542 cVDPV2 Dengue fever 88 930 1 220 Comoros Angola Malawi COVID-19 Chikungunya 33 661 1 123 862 0 3 719 146