50 Years' Observations on Camphor Tree (Cinnamomum Camphora)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phytophthora Ramorum Sudden Oak Death Pathogen
NAME OF SPECIES: Phytophthora ramorum Sudden Oak Death pathogen Synonyms: Common Name: Sudden Oak Death pathogen A. CURRENT STATUS AND DISTRIBUTION I. In Wisconsin? 1. YES NO X 2. Abundance: 3. Geographic Range: 4. Habitat Invaded: 5. Historical Status and Rate of Spread in Wisconsin: 6. Proportion of potential range occupied: II. Invasive in Similar Climate YES NO X Zones United States: In 14 coastal California Counties and in Curry County, Oregon. In nursery in Washington. Canada: Nursery in British Columbia. Europe: Germany, the Netherlands, the United Kingdom, Poland, Spain, France, Belgium, and Sweden. III. Invasive in Similar Habitat YES X NO Types IV. Habitat Affected 1. Habitat affected: this disease thrives in cool, wet climates including areas in coastal California within the fog belt or in low- lying forested areas along stream beds and other bodies of water. Oaks associated with understory species that are susceptible to foliar infections are at higher risk of becoming infected. 2. Host plants: Forty-five hosts are regulated for this disease. These hosts have been found naturally infected by P. ramorum and have had Koch’s postulates completed, reviewed and accepted. Approximately fifty-nine species are associated with Phytophthora ramorum. These species are found naturally infected; P. ramorum has been cultured or detected with PCR but Koch’s postulates have not been completed or documented and reviewed. Northern red oak (Quercus rubra) is considered an associated host. See end of document for complete list of plant hosts. National Risk Model and Map shows susceptible forest types in the mid-Atlantic region of the United States. -

American Materia Medica, Therapeutics and Pharmacognosy
American Materia Medica, Therapeutics and Pharmacognosy Developing the Latest Acquired Knowledge of Drugs, and Especially of the Direct Action of Single Drugs Upon Exact Conditions of Disease, with Especial Reference of the Therapeutics of the Plant Drugs of the Americas. By FINLEY ELLINGWOOD, M.D. 1919 Late Professor of Materia Medica and Therapeutics in Bennett Medical College, Chicago; Professor of Chemistry in Bennett Medical College 1884-1898; Author, and Editor of Ellingwood's Therapeutist; Member National Eclectic Medical Association; American Medical Editors' Association. Abridged to include only the botanical entries, and arranged in alphabetical order by latin names Southwest School of Botanical Medicine P.O. Box 4565, Bisbee, AZ 85603 www.swsbm.com ABIES. Abies canadensis Synonym—Hemlock spruce. CONSTITUENTS— Tannic acid, resin, volatile oil. Canada pitch, or gum hemlock, is the prepared concrete juice of the pinus canadensis. The juice exudes from the tree, and is collected by boiling the bark in water, or boiling the hemlock knots, which are rich in resin. It is composed of one or more resins, and a minute quantity of volatile oil. Canada pitch of commerce is in reddish-brown, brittle masses, of a faint odor, and slight taste. Oil of hemlock is obtained by distilling the branches with water. It is a volatile liquid, having a terebinthinate odor and taste. PREPARATIONS— Canada Pitch Plaster Tincture of the fresh hemlock boughs Tincture of the fresh inner bark. Specific Medicine Pinus. Dose, from five to sixty minims. The hemlock spruce produces three medicines; the gum, used in the form of a plaster as a rubifacient in rheumatism and kindred complaints; the volatile oil—oil of hemlock—or a tincture of the fresh boughs, used as a diuretic in diseases of the urinary organs, and wherever a terebinthinate remedy is indicated; and a tincture of the fresh inner bark, an astringent with specific properties, used locally, and internally in catarrh. -

Evergreen Trees Agonis Flexuosa
Evergreen Trees Agonis flexuosa – Peppermint Willow Graceful willow-like evergreen tree (but without the willows voracious root system) with reddish-brown, deeply furrowed bark to 25’-30’. New leaves and twigs have an attractive reddish cast; clustered small white flowers and brownish fruits are not particularly ornamental. Casaurina stricta – Beefwood Pendulous gray branches; resembles a pine somewhat; tolerates drought, heat, wind, fog. Growth to 20’- 30’. Cinnamomum camphora - Camphor Evergreen trees to 40 feet, with 20-foot spread.. In winter foliage is a shiny yellow green. In early spring new foliage may be pink, red or bronze, depending on tree. Unusually strong structure. Clusters of tiny, fragrant yellow flowers in profusion in May. Geijera parviflora- Australian Willow Evergreen trees with graceful, fine-textured leaves, to 30 feet, 20 feet wide. Main branches weep up and out; little branches hang down. Much of the grace of a willow, much of the toughness of eucalyptus, moderate growth and deep non-invasive roots. Laurus nobilis – Grecian Laurel Slow growth 12-40’. Natural habit is compact, broad-based, often multi-stemmed, gradually tapering cone. Leaves lethery, aromatic. Clusters of small yellow flowers followed by black or purple berries. Magnolia Grandiflora – ‘Little Gem’- Dwarf Southern Magnolia Small tree to 20’ in height. Showy white flowers in the summer. Green glossy leaves. Maytenous boaria - Mayten Evergreen tree with slow to moderate growth to an eventual 30-50 feet, with a 15-foot spread, with long and pendulous branchlets hanging down from branches, giving tree a graceful look. Habit and leaves somewhat like a small scale weeping willow. -

Invasive Trees of Georgia Pub10-14
Pub. No. 39 October 2016 Invasive Trees of Georgia by Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia Georgia has many species of trees. Some are native trees and some have been introduced from outside the state, nation, or continent. Most of Georgia’s trees are well- behaved and easily develop into sustainable shade and street trees. A few tree species have an extrodinary ability to upsurp resources and take over sites from other plants. These trees are called invasive because they effectively invade sites, many times eliminat- ing other species of plants. There are a few tree species native to Georgia which are considered invasive in other parts of the country. These native invasives, may be well-behaved in Georgia, but reproduce and take over sites elsewhere, and so have gained an invasive status from at least one other invasive species list. Table 1. There are hundreds of trees which have been introduced to Georgia landscapes. Some of these exotic / naturalized trees are considered invasive. The selected list of Georgia invasive trees listed here are notorious for growing rampantly and being difficult to eradicate. Table 2. Table 1: Native trees considered invasive in other parts of the country. scientific name common name scientific name common name Acacia farnesiana sweet acacia Myrica cerifera Southern bayberry Acer negundo boxelder Pinus taeda loblolly pine Acer rubrum red maple Populus deltoides Eastern cottonwood Fraxinus americana white ash Prunus serotina black cherry Fraxinus pennsylvanica green ash Robinia pseudoacacia black locust Gleditsia triacanthos honeylocust Toxicodendron vernix poison sumac Juniperus virginiana eastern redcedar The University of Georgia is committed to principles of equal opportunity and affirmative action. -
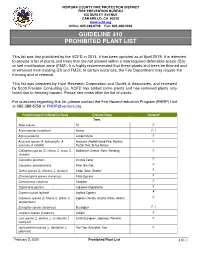
Guideline 410 Prohibited Plant List
VENTURA COUNTY FIRE PROTECTION DISTRICT FIRE PREVENTION BUREAU 165 DURLEY AVENUE CAMARILLO, CA 93010 www.vcfd.org Office: 805-389-9738 Fax: 805-388-4356 GUIDELINE 410 PROHIBITED PLANT LIST This list was first published by the VCFD in 2014. It has been updated as of April 2019. It is intended to provide a list of plants and trees that are not allowed within a new required defensible space (DS) or fuel modification zone (FMZ). It is highly recommended that these plants and trees be thinned and or removed from existing DS and FMZs. In certain instances, the Fire Department may require the thinning and or removal. This list was prepared by Hunt Research Corporation and Dudek & Associates, and reviewed by Scott Franklin Consulting Co, VCFD has added some plants and has removed plants only listed due to freezing hazard. Please see notes after the list of plants. For questions regarding this list, please contact the Fire Hazard reduction Program (FHRP) Unit at 085-389-9759 or [email protected] Prohibited plant list:Botanical Name Common Name Comment* Trees Abies species Fir F Acacia species (numerous) Acacia F, I Agonis juniperina Juniper Myrtle F Araucaria species (A. heterophylla, A. Araucaria (Norfolk Island Pine, Monkey F araucana, A. bidwillii) Puzzle Tree, Bunya Bunya) Callistemon species (C. citrinus, C. rosea, C. Bottlebrush (Lemon, Rose, Weeping) F viminalis) Calocedrus decurrens Incense Cedar F Casuarina cunninghamiana River She-Oak F Cedrus species (C. atlantica, C. deodara) Cedar (Atlas, Deodar) F Chamaecyparis species (numerous) False Cypress F Cinnamomum camphora Camphor F Cryptomeria japonica Japanese Cryptomeria F Cupressocyparis leylandii Leyland Cypress F Cupressus species (C. -

Cinnamomum Camphora) in Eastern Australia Brett J
Chapter 14—Australia’s Ever-changing Forests VI: Proceedings of the Eighth National Conference on Australian Forest History. Brett J. Stubbs et al. (ed.). © 2012, ISBN 978-0-9757906-2-5 Saviour to Scourge: a history of the introduction and spread of the camphor tree (Cinnamomum camphora) in eastern Australia Brett J. Stubbs School of Environmental Science and Management, Southern Cross University, Lismore, NSW 2480 Introduction The 50th year commemorative book of Wyrallah Road Public School, in Lismore, New South Wales, records the lopping of camphor trees bordering the school grounds as a ‘milestone’ for 1999. In similar spirit, at Bexhill Public School near Lismore, on World Forestry Day, 26 March 2004, children were told about the need to remove camphor trees and replace them with native species. Palmwoods State School received a Queensland Arbor Day Award in 2005 for the removal from its grounds of an old camphor tree and the transformation of the stump into an artistic wood carving. It is highly incongruous that trees planted in school yards many years before to provide shade and shelter for children, and to beautify their environment, are now being destroyed, not because they are old and unsafe or have grown inconveniently big, but because of a prevailing attitude akin to revulsion which has developed towards the species. The foregoing are just a few examples of the present-day quest for the elimination of camphor trees from the landscape—a quest which is pursued with as much zeal and vigour in parts of eastern Australia as that to reinstate the region’s lost rainforest. -

Georgia Native Trees Considered Invasive in Other Parts of the Country. Scientific Name Common Name Scientific Name Common Name
Invasive Trees of Georgia Dr. Kim D. Coder, Professor of Tree Biology & Health Care, Warnell School, UGA Georgia has many species of trees. Some are native trees and some have been introduced from outside the state, nation, or continent. Most of Georgia’s trees are well-behaved and easily develop into sustainable shade and street trees. A few tree species have an extrodinary ability to upsurp resources and take over sites from other plants. These trees are called invasive because they effectively invade sites, many times eliminating other species of plants. There are a few tree species native to Georgia which are considered invasive in other parts of the country. These native invasives, may be well-behaved in Georgia, but reproduce and take over sites elsewhere, and so have gained an invasive status from at least one other invasive species list. Table 1. There are hundreds of trees which have been introduced to Georgia landscapes. Some of these exotic / naturalized trees are considered invasive. The selected list of Georgia invasive trees listed here are notorious for growing rampantly and being diffi- cult to eradicate. Table 2. They should not be planted. Table 1: Georgia native trees considered invasive in other parts of the country. scientific name common name scientific name common name Acacia farnesiana sweet acacia Myrica cerifera Southern bayberry Acer negundo boxelder Pinus taeda loblolly pine Acer rubrum red maple Populus deltoides Eastern Fraxinus americana white ash cottonwood Fraxinus pennsylvanica green ash Prunus serotina black cherry Gleditsia triacanthos honeylocust Robinia pseudoacacia black locust Juniperus virginiana eastern Toxicodendron vernix poison sumac redcedar Table 2: Introduced (exotic) tree / shrub species found in Georgia listed at a regional / national level as being ecologically invasive. -

Botanical Name
CITY OF CHULA VISTA URBAN FOREST TREE LIST TREES SUITABLE FOR 3’ X 4’ CUTOUTS OR 3’ TO 4’ PARKWAYS BOTANICAL NAME COMMON NAME HEIGHT WIDTH GROWTH DROUGHT- TOLERANT? Acacia aneura Mulga >20’ >20’ Mod/Fast Yes* Aesculus californica California buckeye 20’ 22’-30’ Moderate Yes* Arbutus ‘Marina’ Arbutus 25’ 25’ Moderate Yes Callistemon citrinus Lemon bottlebrush 25’ 20’ Slow/mod Yes Callistemon viminalis Weeping bottlebrush 25’ 20’ Slow/mod Yes Cercis canadensis Eastern redbud 25’ 25’ Slow/mod Cercis occidentalis Western redbud 20’ 20’ Slow/mod Yes* Chionanthus retusus Chinese fringe tree 20’ 20’ Slow/mod Eriobotrya deflexa Bronze loquat >20’ >20’ Slow/mod Eucalyptus torquata Coral gum 20’ 20’ Moderate Yes Ginkgo biloba Maidenhair 50’ 25’ Fast Hymenosporum flavum Sweetshade 40’ 25’ Slow/mod Lagerstroemia indica Crape myrtle 30’ 20’ Moderate Magnolia grandiflora Southern Magnolia/St. Mary magnolia >20’ >20’ Slow/mod Markhamia hildebrandtii Markhamia 35’ 20’ Slow/mod Maytenus boria Mayten 40’ 30’ Slow Pinus thunbergiana Japanese black pine 20’ 15’ Slow Pittosporum angustifolium Willow pittosporum 20’ 10-15’ Slow Pittosporum rhombifolium Queensland pittosporum 35’ 20’ Moderate Senna splendida (syn. Cassia splendida) Golden wonder senna 12’ 10’ Moderate Yes Stenocarpus sinuatus Firewheel tree 25’ 15’ Moderate Tristaniopsis laurina Water gum 20’ 6-8’. Moderate TREES SUITABLE FOR 5’ X 5’ CUTOUTS OR 6’ TO 8’ PARKWAYS BOTANICAL NAME COMMON NAME HEIGHT WIDTH GROWTH DROUGHT- TOLERANT? Acacia baileyana Bailey acacia 30’ 25’ Fast Yes* Acacia baileyana -

What's a Tree Done for You Lately?
What’s a Tree Done For You Lately? Some common products we get from trees and how they are made Scott Leavengood Director, Oregon Wood Innovation Center, Oregon State University This publication is on-line at http://owic.oregons tate.edu/teachers.php Introduction Products from trees are all around us. Some items are easy to recognize as products we get from trees - This publication will serve as valuable lumber, plywood, and paper, for example. Other background material for several activities in the items such as cellophane, a rayon scarf, and a Project Learning Tree (PLT) Pre K-8 chocolate bar, may not be as easy to recognize as a Environmental Education Activity Guide (1993, product from trees. American Forest Foundation, Washington, D.C.). In particular, the publication is well- This publication will help educators teach students suited to activities 12 and 13, Tree Treasures, about the variety of products we obtain from trees and We All Need Trees, respectively. and how the products are made. It serves as a reference for anyone who wants to know more Number 12 - Tree Treasures about products we obtain from trees. Objectives: 1) identify and categorize products derived from Products from trees may be categorized as: trees, 2) find out which forest products are recyclable • products from wood fiber - for example, paper or reusable, and products and purified cellulose products (rayon, 3) recommend actions for conserving forest cellophane, food additives, and resources. pharmaceuticals); Number 13 - We All Need Trees • products derived -

Biomolecules of Interest Present in the Main Industrial Wood Species Used in Indonesia-A Review
Tech Science Press DOI: 10.32604/jrm.2021.014286 REVIEW Biomolecules of Interest Present in the Main Industrial Wood Species Used in Indonesia-A Review Resa Martha1,2, Mahdi Mubarok1,2, Wayan Darmawan2, Wasrin Syafii2, Stéphane Dumarcay1, Christine Gérardin Charbonnier1 and Philippe Gérardin1,* 1Université de Lorraine, Institut National de Recherche pour l’Agriculture, l’Alimentation et l’Environnement, Laboratoire d'Etudes et de Recherche sur le Matériau Bois, Nancy, France 2Department of Forest Products, Faculty of Forestry and Environment, Institut Pertanian Bogor, Bogor University, Bogor, Indonesia *Corresponding Author: Philippe Gérardin. Email: [email protected] Received: 17 September 2020 Accepted: 20 October 2020 ABSTRACT As a tropical archipelagic country, Indonesia’s forests possess high biodiversity, including its wide variety of wood species. Valorisation of biomolecules released from woody plant extracts has been gaining attractive interests since in the middle of 20th century. This paper focuses on a literature review of the potential valorisation of biomole- cules released from twenty wood species exploited in Indonesia. It has revealed that depending on the natural origin of the wood species studied and harmonized with the ethnobotanical and ethnomedicinal knowledge, the extractives derived from the woody plants have given valuable heritages in the fields of medicines and phar- macology. The families of the bioactive compounds found in the extracts mainly consisted of flavonoids, stilbenes, stilbenoids, lignans, tannins, simple phenols, terpenes, terpenoids, alkaloids, quinones, and saponins. In addition, biological or pharmacological activities of the extracts/isolated phytochemicals were recorded to have antioxidant, antimicrobial, antifungal, anti-inflammatory, anti-diabetes, anti-dysentery, anticancer, analgesic, anti-malaria, and anti-Alzheimer activities. -

Panhandle Regional Working Group
Panhandle Regional Working Group Florida Caverns Invasive Exotic Plant Control County: Jackson PCL: Florida Caverns State Park PCL Size: 1,279.25 acres Project Manager: Florida Park Service Mark Ludlow, Park Biologist 3345 Caverns Road, Marianna, Florida 32446 Phone: 850-482-9289, Fax: 850-482-9114 E-mail: [email protected] Project ID: PH-015 Project Size: 52 acres Fiscal Year 01/02 Project Cost: $18,034.59 Florida Caverns State Park is located off Highway 166, about three miles north of Marianna, in Jackson County. The majority of the park is Chipola River floodplain forest and upland mixed forest. Approximately 30 acres in the old federal fish hatchery area on the west side of the park were disturbed in the 1930s for hatchery ponds. The ponds, abandoned in the mid-1940s, were re-colonized by native hardwoods mixed with exotic shrubs and trees. Chinese privet was the most abundant exotic plant on the site with numerous small seedlings adjacent to the staff residence buildings. Target Plants Common Name FLEPPC Rank Treatment Herbicide Albizia julibrissin mimosa Category I basal bark Garlon 4 Cinnamomum camphora camphor tree Category I basal bark Garlon 4 Nandina domestica heavenly bamboo Category I basal bark Garlon 4 Ligustrum sinense Chinese privet Category I basal bark Garlon 4 Elaeagnus pungens silverthorn Category II basal bark Garlon 4 Backpack sprayers are commonly used by applicators. Especially in dense ‘jungle’ conditions. 45 Maclay Gardens Invasive Exotic Plant Control County: Leon PCL: Maclay Gardens State Park PCL Size: 1,779.15 acres Project Manager: Florida Park Service Beth Weidner, Park Manager 3540 Thomasville Road, Tallahassee, FL 32304 Phone: 850-487- 4115, FAX: 850- 487- 8808 E-mail: [email protected] Project ID: PH-013 Project Size: 200 acres Fiscal Year 01/02 Project Cost: $133,551.08 Maclay Gardens State Park is located on US Highway 319 in Tallahassee. -

Cinnamomum Camphora, Camphor Laurel Most Toxic Chemotypes.’
Advice to the Minister for the Environment and Heritage from the Threatened Species Scientific Committee (TSSC) on Amendments to the List of Key Threatening Processes under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) 1. Name and description of the threatening process ‘Cinnamomum camphora, Camphor Laurel most toxic chemotypes.’ Camphor Laurel is a large evergreen tree that was introduced into Australia following European settlement. The species is an invasive woody weed capable of replacing native trees along watercourses and on soil types which formerly supported rainforests. The infestation and spread of Camphor Laurel is aided by birds dispersing seed and its spread is particularly prevalent in disturbed landscapes. The nomination focuses on the claimed toxic effects of certain forms of the species. It proposes that the most toxic forms of Camphor Laurel contain compounds in their fruit, seeds, leaves, roots and bark which have caused declines in native species throughout northern New South Wales and southeastern Queensland. The nomination highlights that Camphor Laurel trees can exist in up to nine different chemical forms. The nominator uses the description ‘most toxic chemotypes1’ to refer to those Camphor Laurel trees with the greatest levels of toxic compounds. It is asserted that the hybridisation of various forms of Camphor Laurel has assisted in the proliferation of the more toxic chemotypes and that where the most toxic chemotypes of Camphor Laurel are present, loss of regional biodiversity