Pulmonary Valve Guideline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 12 the Cardiovascular System: the Heart Pages
CHAPTER 12 THE CARDIOVASCULAR SYSTEM: THE HEART PAGES 388 - 411 LOCATION & GENERAL FEATURES OF THE HEART TWO CIRCUIT CIRCULATORY SYSTEM DIVISIONS OF THE HEART FOUR CHAMBERS Right Atrium Left Atrium Receives blood from Receives blood from the systemic circuit the pulmonary circuit FOUR CHAMBERS Right Ventricle Left Ventricle Ejects blood into the Ejects blood into the pulmonary circuit systemic circuit FOUR VALVES –ATRIOVENTRICULAR VALVES Right Atrioventricular Left Atrioventricular Valve (AV) Valve (AV) Tricuspid Valve Bicuspid Valve and Mitral Valve FOUR VALVES –SEMILUNAR VALVES Pulmonary valve Aortic Valve Guards entrance to Guards entrance to the pulmonary trunk the aorta FLOW OF BLOOD MAJOR VEINS AND ARTERIES AROUND THE HEART • Arteries carry blood AWAY from the heart • Veins allow blood to VISIT the heart MAJOR VEINS AND ARTERIES ON THE HEART Coronary Circulation – Supplies blood to the muscle tissue of the heart ARTERIES Elastic artery: Large, resilient vessels. pulmonary trunk and aorta Muscular artery: Medium-sized arteries. They distribute blood to skeletal muscles and internal organs. external carotid artery of the neck Arteriole: Smallest of arteries. Lead into capillaries VEINS Large veins: Largest of the veins. Superior and Inferior Vena Cava Medium-sized veins: Medium sized veins. Pulmonary veins Venules: the smallest type of vein. Lead into capillaries CAPILLARIES Exchange of molecules between blood and interstitial fluid. FLOW OF BLOOD THROUGH HEART TISSUES OF THE HEART THE HEART WALL Pericardium Outermost layer Serous membrane Myocardium Middle layer Thick muscle layer Endocardium Inner lining of pumping chambers Continuous with endothelium CARDIAC MUSCLE Depend on oxygen to obtain energy Abundant in mitochondria In contact with several other cardiac muscles Intercalated disks – interlocking membranes of adjacent cells Desmosomes Gap junctions CONNECTIVE TISSUE Wrap around each cardiac muscle cell and tie together adjacent cells. -

Note: Before Reading the Specific Defect
Pulmonary Valve Stenosis and Regurgitation What is it? The pulmonary valve opens to let blood flow from the right ventricle to the lungs. Narrowing of the pulmonary valve, known as valvular pulmonary stenosis (PS) causes the right ventricle to pump harder to get blood past the blockage. Normally the pulmonary valve has three cusps. If these cusps are malformed, the valve may become narrowed (stenotic) or leaky (regurgitant or insufficiency). Pulmonary valve stenosis is much more common than regurgitation. The stenosis and regurgitation or both can be mild, moderate or severe. What causes it? In most cases, the cause isn’t known. It’s a common type of heart defect. Babies born to mothers who had rubella (German measles) during pregnancy were more likely to develop pulmonary stenosis along with deafness and patent ductus arteriosus. Some patients can have other heart defects along with PS. How does it affect the heart? Normally the right side of the heart pumps blood to the lungs. In a person with PS, the pressure in the right-heart pumping chamber (right ventricle) is much higher than normal and the heart must work harder to pump blood out into the lung arteries. Over time this can cause damage to the overworked heart muscle. When the valve is regurgitant it can cause the right ventricle to enlarge. How does the PS affect me? If the PS is severe, adults may complain of chest pain, exercise intolerance or have no symptoms. Cyanosis is rare and usually occurs only when there is an atrial or ventricular septal defect as well. -

Glossary of Commonly Used Heart and Vascular Terms
Glossary of Commonly Used Terms • Angina: Chest pain that occurs when an area of the heart muscle does not receive enough oxygen-rich blood. • Aorta: The largest artery in the body, it receives blood from the heart that has been oxygenated in the lungs, and delivers this blood to the body and brain. • Aortic Stenosis: A progressive disease that affects the aortic valve of the heart. • Aortic valve: The heart valve that regulates the one-way flow of blood from the left ventricle to the aorta. • Arteries: The blood vessels that carry oxygen-rich blood away from the heart and lungs. • Atria: The two upper chambers of the heart that receive blood returning from the body. • Balloon valvuloplasty: A procedure performed to open a narrowed heart valve using a thin tube called a catheter with a small balloon at its tip. The catheter is inserted through a small incision in the groin and then threaded up to the opening of the narrowed heart valve. The balloon is then inflated to stretch the valve open and relieve valve obstruction. • Bi-leaflet: A valve that has two leaflets that regulate the flow of blood. A normal aortic valve has three leaflets. • Calcification: A disease state in which calcium from the blood collects in the body tissues. When this occurs on the leaflets of the heart’s valves, it may cause them to harden and reduce their ability to open and close properly. • Cardiopulmonary bypass: Bypass of the heart and lungs. During this technique, which is often used during heart surgery, a heart-lung machine temporarily takes over the function of the heart and lungs. -

PHV Short.Indd
Anatomy & physiology: The heart Cardiac electrical conductance A Sinoatrial (SA) node D Right bundle branch B Atrioventricular (AV) node E Left bundle branch A C Atrioventricular bundle of His F Purkinje fibres The sinoatrial (SA) node is the heart's natural pacemaker, B containing cells that generate electrical impulses which spread F through the atria, triggering contraction of the atria, forcing blood into the ventricles, and stimulating the atrioventricular A (AV) node. E The AV node is linked with the atrioventricular bundle of His C which transmits impulses to the left and right bundle branches and then to the Purkinje fibres which surround the ventricles. The electrical impulses travel through the Purkinje fibres D causing the ventricles to contract and blood forced out of the heart. F Electrocardiogram (ECG) Heart construction The heart is a muscular, cone-shaped organ located behind The ECG traces the course of the cardiac impulse by the sternum and is approximately the same size as the recording the change in electrical potential on the surface of patient's closed fist. the body. Various parts of the ECG are associated with the travel of electrical impulses though the heart. The heart walls are made up the three structures: the pericardium, myocardium and endocardium. The P wave represents atrial depolarisation which causes the atria to contract. The pericardium is a thick fibrous membrane which surrounds the heart. Its function is to anchor the heart and Q is when the impulses arrive at the atrioventricular (AV) prevents over distension (expanding too much). node. The myocardium is the central layer of the heart and is The QRS complex represents ventricular depolarisation and formed from cardiac muscle tissue, it is this muscle which atrial repolarisation (ventricles contract and atria relax and provides the force which pumps the blood around the body. -

Pulmonary Valve Preservation Strategies for Tetralogy of Fallot Repair Constantine Mavroudis, MD
Pulmonary Valve Preservation Strategies for Tetralogy of Fallot Repair Constantine Mavroudis, MD he optimal repair for tetralogy of Fallot is challenged by techniques and acceptance of high intraoperative right ventri- Tthe management of the small right ventricular outflow cular to left ventricular pressure ratios of 0.7 or less.6-10 tract (RVOT), which was initially solved by John Kirklin who Transannular patches, when necessary, involved a strategy of determined that the absence of the pulmonary valve could transatrial RVOT resection, transatrial VSD closure, and be well tolerated owing to low pulmonary artery pressure limited ventriculotomy, oftentimes not extending the incision and preserved right ventricular function.1 This assumption more than 1-2 cm. Under these circumstances, transannular was affirmed in a significant number of patients who tolerated patches were intentionally downsized and reconstructed the persistent effects of right ventricular volume overload for to result in right ventricular gradients of approximately many years. In a significant number of postoperative patients, 20 mm Hg.11,12 however, long-term complications of pulmonary regurgitation, More comprehensive valve-sparing techniques that in- residual ventricular septal defects (VSD), distal pulmonary cluded a supravalvar pantaloon autologous pericardial patch artery stenosis, right ventricular dysfunction, atrial or ven- for supravalvar pulmonary stenosis were highlighted by – tricular arrhythmias, and exercise intolerance2-5 required several authors.8 10 These -

Incidental Cardiac and Pericardial Abnormalities on Chest CT
PICTORIAL ESSAY Incidental Cardiac and Pericardial Abnormalities on Chest CT Soo-Hyun Lee, MD,*w Joon Beom Seo, MD,* Joon-Won Kang, MD,* Eun Jin Chae, MD,* Seong Hoon Park, MD,* and Tae-Hwan Lim, MD* of an acute myocardial infarction results in a series of Objective: Although there has been an increasing interest in changes in the left ventricular (LV) myocardium, char- diagnosing cardiac and pericardial diseases using electrocardio- acterized by acute ischemia, myocardial death, and gram-gated cardiac computed tomography (CT), radiologists chronic remodeling.1 Although early studies performed tend to neglect or overlook many incidentally detected on conventional, single-slice, whole-body CT scanners, abnormalities on conventional, nongated chest CT. The demonstrated that acute myocardial infarction could be objective of this study is to describe the imaging appearance identified as a region of lower attenuation than normal and clinical significance of such cardiac or pericardial diseases. enhanced myocardium, diagnostic accuracy using the Conclusions: Recognition and detection of various cardiac and conventional CT is very low in diagnosing the acute pericardial abnormalities on chest CT as the evaluation of myocardial events as the cause of acute chest pain. noncardiac disease is important for its early diagnosis and However, incidental detection of coronary calcification 2,3 proper management. identifies individuals at risk for acute coronary events. Coronary artery calcification correlates highly with Key Words: CT, thoracic, heart, pericardium plaque burden but its relationship to plaque instability (J Thorac Imaging 2008;23:216–226) is low (Fig. 1). A mild degree of calcification characterizes patients with acute coronary events, whereas diffused high-attenuation calcific plaques are associated with chronic coronary events.4 dvances in computed tomography (CT) and magnetic LV myocardial aneurysms are relatively common resonance imaging have brought renewed interest to A sequelae of transmural myocardial infarction. -

1. Right Coronary 2. Left Anterior Descending 3. Left
1. RIGHT CORONARY 2. LEFT ANTERIOR DESCENDING 3. LEFT CIRCUMFLEX 4. SUPERIOR VENA CAVA 5. INFERIOR VENA CAVA 6. AORTA 7. PULMONARY ARTERY 8. PULMONARY VEIN 9. RIGHT ATRIUM 10. RIGHT VENTRICLE 11. LEFT ATRIUM 12. LEFT VENTRICLE 13. PAPILLARY MUSCLES 14. CHORDAE TENDINEAE 15. TRICUSPID VALVE 16. MITRAL VALVE 17. PULMONARY VALVE Coronary Arteries Because the heart is composed primarily of cardiac muscle tissue that continuously contracts and relaxes, it must have a constant supply of oxygen and nutrients. The coronary arteries are the network of blood vessels that carry oxygen- and nutrient-rich blood to the cardiac muscle tissue. The blood leaving the left ventricle exits through the aorta, the body’s main artery. Two coronary arteries, referred to as the "left" and "right" coronary arteries, emerge from the beginning of the aorta, near the top of the heart. The initial segment of the left coronary artery is called the left main coronary. This blood vessel is approximately the width of a soda straw and is less than an inch long. It branches into two slightly smaller arteries: the left anterior descending coronary artery and the left circumflex coronary artery. The left anterior descending coronary artery is embedded in the surface of the front side of the heart. The left circumflex coronary artery circles around the left side of the heart and is embedded in the surface of the back of the heart. Just like branches on a tree, the coronary arteries branch into progressively smaller vessels. The larger vessels travel along the surface of the heart; however, the smaller branches penetrate the heart muscle. -

The Heart and Circulation ;
7/28/2020 The Heart and Circulation ; Offered to you by: The Heart and Circulation To understand heart disease and its effects on your body, it may help to learn more about your heart. This booklet explains the structure of the heart and blood vessels and how they work. Words in bold are explained in a word list at the end of the resource. Objectives: This information may help you: Identify the size and location of your heart. Identify the three layers of the heart. Identify the four chambers of your heart. Explain the purpose of the valves. Describe the three main kinds of blood vessels. Explain the role of coronary (heart) arteries in the functioning of your heart. Discuss the difference between the systolic and diastolic blood pressure. Trace the basic route of your heart’s electrical conduction system on the diagram provided. LOCATION AND SIZE OF THE HEART Your heart is located under your rib cage beneath and to the left of your breastbone (sternum) (figure 1). About the size of your fist, the heart is a hollow, muscular organ that weighs less than a pound. Hardworking and powerful, the heart pumps blood to all parts of the body — to every cell, muscle, bone, and organ. https://askmayoexpert.mayoclinic.org/patient-education/topic/clinical-answers/gnt-20238619 1/13 7/28/2020 The Heart and Circulation LAYERS OF THE HEART The heart lies inside a protective sac of fibrous tissue called the pericardium (figure 2). The heart itself has three layers of tissue: the epicardium, the myocardium, and the endocardium. -

CASE REPORT Combined Mitral and Pulmonary Valve Stenosis R
Br Heart J: first published as 10.1136/hrt.28.1.139 on 1 January 1966. Downloaded from Brit. HeartJ., 1966, 28, 139. CASE REPORT Combined Mitral and Pulmonary Valve Stenosis R. M. McCREDIE* AND J. G. RICHARDS From the Hallstrom Institute of Cardiology, Royal Prince Alfred Hospital, Sydney, Australia The incidence of pulmonary valve involvement in an enlarged heart with prominence of the left atrium and rheumatic heart disease is estimated at less than 2 per of the main pulmonary artery. There was early septal cent in most necropsy series (Cabot, 1926; Clawson, cedema at the lung bases and diminished pulmonary 1940), but the occurrence of hmmodynamically signifi- vascular markings in the lower zones. This was thought cant rheumatic pulmonary stenosis is very rare. Of the to be consistent with mitral valve disease with pulmonary few reported cases of pulmonary stenosis in chronic hypertension, but it was noted that the pulmonary artery rheumatic heart disease, most have been cases of quadri- was larger than is usually seen in this situation. Heemo- valvular stenosis recognized only at necropsy (Shattuck, globin was 12-7 g./100 ml., white cell count 9200, E.S.R. 1891; Schwartz, and Shelling, 1931; McGuire and Mc- 37 mm. in 1 hour (Westergren), blood urea 42 mg./ Namara, 1937; Clawson, 1940; Hardin and Daniels, 100 ml.; negative investigations included urinary 5- 1942). The association of severe pulmonary stenosis hydroxy-indole acetic acid, anti-streptolysin titre, with severe mitral stenosis in the absence of evidence of Casoni, and Wasserman reactions. organic aortic or tricuspid valve involvement has not Cardiac catheterization was performed in September been reported. -

Anatomy and Physiology of the Cardiovascular System
Chapter © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC 5 NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Anatomy© Jonesand & Physiology Bartlett Learning, LLC of © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION the Cardiovascular System © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION OUTLINE Aortic arch: The second section of the aorta; it branches into Introduction the brachiocephalic trunk, left common carotid artery, and The Heart left subclavian artery. Structures of the Heart Aortic valve: Located at the base of the aorta, the aortic Conduction System© Jones & Bartlett Learning, LLCvalve has three cusps and opens© Jonesto allow blood & Bartlett to leave the Learning, LLC Functions of the HeartNOT FOR SALE OR DISTRIBUTIONleft ventricle during contraction.NOT FOR SALE OR DISTRIBUTION The Blood Vessels and Circulation Arteries: Elastic vessels able to carry blood away from the Blood Vessels heart under high pressure. Blood Pressure Arterioles: Subdivisions of arteries; they are thinner and have Blood Circulation muscles that are innervated by the sympathetic nervous Summary© Jones & Bartlett Learning, LLC system. © Jones & Bartlett Learning, LLC Atria: The upper chambers of the heart; they receive blood CriticalNOT Thinking FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Websites returning to the heart. Review Questions Atrioventricular node (AV node): A mass of specialized tissue located in the inferior interatrial septum beneath OBJECTIVES the endocardium; it provides the only normal conduction pathway between the atrial and ventricular syncytia. -
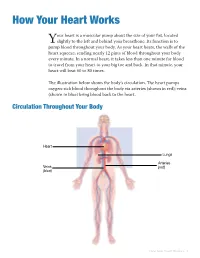
How Your Heart Works
How Your Heart Works our heart is a muscular pump about the size of your fist, located slightly to the left and behind your breastbone. Its function is to Ypump blood throughout your body. As your heart beats, the walls of the heart squeeze, sending nearly 12 pints of blood throughout your body every minute. In a normal heart, it takes less than one minute for blood to travel from your heart to your big toe and back. In that minute, your heart will beat 60 to 80 times. The illustration below shows the body’s circulation. The heart pumps oxygen-rich blood throughout the body via arteries (shown in red); veins (shown in blue) bring blood back to the heart. Circulation Throughout Your Body Heart Lungs Arteries Veins (red) (blue) How Your Heart Works • 1 Heart Anatomy The heart has two sides, separated by an inner wall called theseptum . The right side of the heart pumps blood to the lungs to pick up oxygen. The left side of the heart receives the oxygen-rich blood from the lungs and pumps it to the body. The heart has four chambers and four valves and is connected to various blood vessels. Veins are blood vessels that carry blood from the body to the heart. Arteries are blood vessels that carry blood away from the heart to the body. The illustration shows a cross-section of a healthy heart with its inside structures. The explanations of these structures are listed on the next page. Aorta (to body) Superior vena cava (from upper body) Pulmonary artery Left pulmonary arteries Right pulmonary (to left lung) arteries (to right lung) Left pulmonary Right pulmonary veins (from left lung) veins (from right Left Heart: Right Heart: Left atrium Right atrium Aortic valve Pulmonary valve Mitral valve Tricuspid valve Left ventricle Right ventricle Septum Inferior vena cava (from lower body) Aorta 2 • Heart Surgery: A Guide for Patients and Their Families • Michigan Medicine Heart Chambers The heart has four chambers. -

Morphology of Right Atrioventricular Valve Annulus and Leaflets in Autopsy Specimens
Jemds.com Original Research Article Morphology of Right Atrioventricular Valve Annulus and Leaflets in Autopsy Specimens Raniprabha Sukumaran1, Maheswary Thampi Santhakumary2 1Assistant Professor, Department of Anatomy, Government Medical College, Kottayam, Kerala, India. 2Associate Professor, Department of Anatomy, Government Medical College, Kottayam, Kerala, India. ABSTRACT BACKGROUND Most tricuspid valve conditions are mechanical problems that will require surgery Corresponding Author: Dr. Maheswary Thampi Santhakumary, like valve repair and valve replacement. The proper size of the valve prosthesis and Associate Professor, the size of the annuloplasty band are determined by the measurements of the valve Department of Anatomy, annulus and leaflets. The present study is aimed to provide normal measurements of Government Medical College, the tricuspid valve that would help cardiac surgeons in proper planning and Kottayam, Kerala, India. execution of tricuspid valve surgeries. E-mail: [email protected] METHODS DOI: 10.14260/jemds/2019/552 This descriptive study was conducted in 60 adult human hearts of both sexes during Financial or Other Competing Interests: medico legal autopsy in the Department of Forensic Medicine, GMC Kottayam from None. November 2018 to April 2019. The heart specimens, free from any pathology, variation, were dissected. The diameter, circumference of the annulus and the length How to Cite This Article: and height of the leaflets were measured. The range, mean, standard deviation and Sukumaran R, Santhakumary MT. coefficient of variation of each parameter were calculated. Morphology of right atrioventricular valve annulus and leaflets in autopsy specimens. RESULTS J. Evolution Med. Dent. Sci. 2019;8(32): In the present study, the circumference of the tricuspid valve measured 11.65+/- 1.28 2534-2538, DOI: cms.