HOW to CREATE a PRODUCT QUALITY ROADMAP Ebook TABLE of CONTENTS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
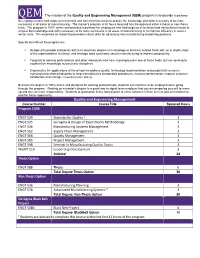
QEM the Mission of the Quality and Engineering Management
QEM The mission of the Quality and Engineering Management (QEM) program is to provide a pathway for employees who hold degrees in technical and non-technical areas to acquire the knowledge and skills necessary to be more successful in all areas of manufacturing. The master’s program is 30 hours long and has the option of either a thesis or non-thesis track. The program is 100% online and provides a pathway for employees who hold degrees in technical and nontechnical areas to acquire the knowledge and skills necessary to be more successful in all areas of manufacturing or to improve efficiency in service sector units. The emphasis on leadership provides critical skills for advancing into manufacturing leadership positions. Specific benefits of the program are: Designed to provide individuals with baccalaureate degrees in technology or business related fields with an in-depth study of the organizational, technical, and strategic tools commonly used in manufacturing to improve productivity Targeted to working professionals and other individuals who have a background in one of these fields, but are seeking to expand their knowledge across these disciplines Emphasizes the applications of these tools to address quality, technology implementation and productivity issues in manufacturing related industries to help manufacturers standardize procedures, measure performance, improve customer satisfaction and manage resources more wisely Because the degree is 100% online and designed for working professionals, students can continue to be employed while going through the program. Working on a master’s degree is a great way to signal to an employer that you are preparing yourself to move up and take on more responsibility. -

Defining the Quality of Business Processes
Defining the Quality of Business Processes Robert Heinrich1 and Barbara Paech2 Abstract: Business process models are used to gain a joint understanding of complex processes. Often they are applied in change projects where either the supporting IT or the processes themselves or both are to be improved. So it is an important question to assess the quality of the modeled business processes. However, so far there is no standard definition of the quality of a business process. Furthermore, business process models are not tuned to capture quality aspects. The goal of our work is to collect important quality characteristics and attributes of processes and to enhance business process modeling languages with means to express these attributes. This paper is a first step in this direction. We define a first set of quality characteristics, attributes and measures for these attributes in a business process. As an example we evaluate how well these measures can be expressed in a BPMN model. 1Introduction Business process models aim at providing a joint understanding of business processes. Therefore, they typically cover information about structure and behavior like description of activities or decisions within the process. But they do not aim at providing quality information. Quality information, for example, is information about the reliability or the usability of a business process. That information is of high interest for organizations because business process models are often used in change projects where either the supporting IT or the processes themselves or both are to be enhanced. Furthermore, the quality of a business process highly affects the success of an organization. -

Quality Management
DEPARTMENT OF HEALTH AND HUMAN SERVICES ENTERPRISE PERFORMANCE LIFE CYCLE FRAMEWORK <OPDIV Logo> PPPRRRAAACCCTTTIIICCCEEESSS GGGUUUIIIDDDEEE QUALITY MANAGEMENT Issue Date: <mm/dd/yyyy> Revision Date: <mm/dd/yyyy> Document Purpose This Practices Guides is a brief document that provides an overview describing the best practices, activities, attributes, and related templates, tools, information, and key terminology of industry-leading project management practices and their accompanying project management templates. This guide is focused on practices that should be applied to ensure the project will satisfy business needs, objectives, and requirements. Background The Department of Health and Human Services (HHS) Enterprise Performance Life Cycle (EPLC) is a framework to enhance Information Technology (IT) governance through rigorous application of sound investment and project management principles and industry’s best practices. The EPLC provides the context for the governance process and describes interdependencies between its project management, investment management, and capital planning components. The Quality Management Plan is an important component of a Project Management Plan deliverable. The discipline of quality management complements project management with its focus on customer satisfaction, prevention of defects over inspection, management responsibility, and continuous improvement. Practice Overview Project Managers should assume responsibility for the development of quality policies for the project and the coordination of related activities, in compliance with any organizational and/or regulatory standards. A Quality Management Plan documents this information and describes the authorities, policies, tools and techniques that are specific to ensuring project excellence, reducing cost and eliminating unnecessary corrections and/or changes. It is important to note that the concept of quality does not necessarily require perfection. -

Using Quality Assurance Standards
Using Quality Assurance Standards Don’t assume quality, ensure quality Learning Objectives . At the end of this module, you will be able to: – Identify the difference between Quality Assurance (QA) and Quality Control (QC). – Identify ISO 9000 and Six Sigma programs. – List some considerations for implementing a quality assurance program. FDIC OMWI Education Module: Using Quality Assurance Standards 2 About FDIC Small Business Resource Effort . The Federal Deposit Insurance Corporation (FDIC) recognizes the important contributions made by small, veteran, and minority and women-owned businesses to our economy. For that reason, we strive to provide small businesses with opportunities to contract with the FDIC. In furtherance of this goal, the FDIC has initiated the FDIC Small Business Resource Effort to assist the small vendors that provide products, services, and solutions to the FDIC. The objective of the Small Business Resource Effort is to provide information and the tools small vendors need to become better positioned to compete for contracts and subcontracts at the FDIC. To achieve this objective, the Small Business Resource Effort references outside resources critical for qualified vendors, leverages technology to provide education according to perceived needs, and offers connectivity through resourcing, accessibility, counseling, coaching, and guidance where applicable. This product was developed by the FDIC Office of Minority and Women Inclusion (OMWI). OMWI has responsibility for oversight of the Small Business Resource Effort. FDIC OMWI Education Module: Using Quality Assurance Standards 3 Executive Summary . Every customer wants a quality product, and the government is no exception. With the government or its prime contractors, "quality will be assumed." . -

Overview of Program Evaluation and Quality Improvement
Overview of Program Evaluation and Quality Improvement Raymond. A. Howard, MBA, MPH Maria Lourdes F. Reyes, MD, MPH Learning Objectives . Importance and meaning of evaluation as an integrated component of program design, planning and implementation. Two types of evaluation and their respective values in program evaluation . Use of Logic Models . Application of a Quality Improvement (QI) Process . Example of a Successful QI Approach What is Evaluation? Program evaluation is a systematic way to gain insight as to how your program or project is being implemented from an operational and service perspective. “If you don’t know where you are going, you might wind up some place else” Yogi Berra Two Types of Evaluation Process Evaluation (Formative) . Monitors the program’s implementation from the beginning of the program or project through the end . Seeks to Answer: Is the program being carried out according to plan? Is it working the way its supposed to work? How can the program implementation be improved? Two Types of Evaluation (Cont’d) Outcome Evaluation (Summative) . Is usually done at the conclusion of the program (although the data collection is done throughout the program). Seeks to Answer Did the program achieve its purpose? (i.e. did it have the intended/desired impact or did it benefit to the participants in the way that was expected?) Using Logic Models: The maps to your programming journey. .Outlines how the “journey” is supposed to proceed. .Provides a sense of the initial direction you want to take. .Allows you to check your progress. .Informs the need for a course correction. Oh……You mean that there’s a method to this madness? Basic Framework for Program Logic Model Start Here Goal: Inputs Activities Outputs Initial or Intermediate Long-Term Short-term Outcomes Outcomes Outcomes A program’s What a Products of a Changes in Changes in Changes in the resources and program does program’s participants’ participants’ condition or constraints/bar with its inputs to activities. -

Quality Management System (QMS) for Apis
Quality Management System (QMS) for Active Pharmaceutical Ingredients (API) Manufacturers integrating GMP (ICH Q7a) into ISO (9001: 2000) September 2005 APIC / CEFIC Quality Management System - integrating GMP (ICH Q7a) into ISO (9001: 2000) Page 2 of 73 Table of Contents I. Introduction II. Objective and Scope III. Quality Management Systems for API manufacturers 1. Quality Management System 2. Management responsibility 3. Resource management 4. Product realization (Manufacturing Operations) 5. Measurement, analysis and improvement (Evaluation Activities) IV. Supplementary Information 1. Identification of system approaches in Q7a 2. Description of processes 3. Structure of a Quality Manual 4. Cross-reference of APIC QMS documents (from “old” to “new”) 5. Assistance for implementation of a QMS 6. Matrix GMP(Q7a) / ISO (9001:2000) V. Glossary VI. Abbreviations VII. References VIII. Acknowledgements APIC / CEFIC Quality Management System - integrating GMP (ICH Q7a) into ISO (9001: 2000) Page 3 of 73 I. Introduction The changing regulatory environment In a Science Board Meeting held in November 2001, FDA raised some concerns regarding the efficiency of the pharmaceutical industry. The factors contributing to this situation were identified as follows: • Pharmaceuticals are complex, multivariate physicochemical systems that are - Often treated (during development) as univariate systems (one-factor-at-a-time, trial- and-error experimentation) - Physical properties of materials normally not well characterized - Equipment selection based on tradition - Process factors are not well understood • Development is done under time crunch • Post approval changes require regulatory oversight It was said that a higher efficiency is required in order to provide high quality drugs to the market in a timely manner, to successfully take advantage of the new drug development opportunities offered by advances in chemistry and biology. -

Quality Engineering in The
QUALITY ENGINEERING IN THE NEW A vision and R&D update from Accenture Labs and Accenture Testing Services QUALITY ENGINEERING WITH BUSINESS PURPOSE In recent years, traditional approaches to application testing have been significantly disrupted with the advent of DevOps and intelligent automation, as well as proliferation of digital applications. As delivery timelines have shrunk from months to weeks and now days, testing has shifted both left and right in the software development lifecycle. DevOps and agile have merged development and testing into a single continuous activity. Testing has evolved to quality engineering that begins upfront as part of initial application planning and establishes a continuous feedback loop to anticipate and act on the unknown. But to truly understand the scale of evolution from testing to quality engineering, we need to recognize how data has changed software development itself. The power of data goes well beyond fueling automation use cases and AI learning datasets for repetitive development and testing tasks. The massive amounts of data users generate every day is now elevating quality engineers to predict risk, identify opportunities, increase speed and agility and minimize technical debt. With this vast influx of data, the quality engineer’s role has become far more exciting but also complex. It will further evolve as we move towards AI, edge computing and the massive IoT end datasets which require machine-to-machine (M2M) communications with complete autonomy and failsafe protection. Accenture has long been at the forefront of testing innovation, and now we are leading our clients into the world of quality engineering at scale. -

Sample Marketing Plan © Zayats-And-Zayats/Shutterstock.Com
Sample Marketing Plan © zayats-and-zayats/Shutterstock.com This sample marketing plan for a hypothetical company illustrates how the marketing plan- ning process described in Chapter 2 might be implemented. If you are asked to create a mar- keting plan, this model may be a helpful guide, along with the concepts in Chapter 2 . Star Software Inc. Marketing Plan I. EXECUTIVE SUMMARY 1 1 The Executive Summary, one of the most frequently read Star Software Inc. is a small, family-owned corporation in the first year of a transition from components of a marketing plan, is a synopsis of the mar- first-generation to second-generation leadership. Star Software sells custom-made calendar keting plan. Although it does not programs and related items to about 400 businesses, which use the software mainly for provide detailed information, it promotion. As Star’s business is highly seasonal, its 18 employees face scheduling challenges, does present an overview of the plan so readers can identify key with greatest demand during October, November, and December. In other months, the issues pertaining to their roles in equipment and staff are sometimes idle. A major challenge facing Star Software is how to the planning and implementation increase profits and make better use of its resources during the off-season. processes. Although this is the fi rst section in a marketing plan, An evaluation of the company’s internal strengths and weaknesses and external oppor- it is usually written last. tunities and threats served as the foundation for this strategic analysis and marketing plan. The plan focuses on the company’s growth strategy, suggesting ways it can build on existing customer relationships, and on the development of new products and/or services targeted to specific customer niches. -

Quality Assurance and Quality Control
November 2018 Vol. 02, No. 23 Quality Assurance and Quality Control ppendix E of the DRM, A/E Submission Requirements, standards have been met. The QC team should report outlines the content and quality requirements for design deficiencies so that they can be addressed in an improve QA A document submissions. To meet these requirements it is process. necessary for the A/E to utilize Quality Assurance (QA) and QA and QC During Construction Quality Control (QC) procedures. QA and QC are separate and The definition of ‘quality’ of construction can vary by project. distinct activities, both of which are necessary to ensure the Generally, it means meeting project parameters (such as cost, quality of an end product, including design documents and schedule, and contractual and regulatory requirements), construction. avoiding disputes, meeting the design intent of the construction QA is the conscious planning and implementation of systems and documents, and producing a facility that meets the owner’s procedures to ensure that a process is carried out with a high expectations and performs as intended. probability of success. QA is typically proactive, and begins at the Every project should have a written QA plan which outlines the onset of a process. QA focuses on failure prevention. required processes, standards and policies. The QA plan must QC is the systematic checking of an end product to identify define the efficient and organized management of all aspects of failures and either correct or eliminate them. QC is typically the construction process, including personnel, information and reactive, and conducted at the end of a process. -

Quality Manufacturing a Blockbuster Opportunity for Pharmaceuticals
Quality manufacturing A blockbuster opportunity for pharmaceuticals An Economist Intelligence Unit white paper written in co-operation with Oracle Quality manufacturing A blockbuster opportunity for pharmaceuticals Preface Quality manufacturing: a blockbuster opportunity for pharmaceuticals is an Economist Intelligence Unit white paper, sponsored by Oracle. The Economist Intelligence Unit bears sole responsibility for this report. The Economist Intelligence Unit’s editorial team conducted the interviews, wrote and edited the report. The findings and views expressed in this report do not necessarily reflect the views of the sponsor. John du Pre Gauntt is the author of the report. Our research drew on desk research and in-depth interviews with senior executives and government officials in the field of pharmaceuticals. Our thanks are due to the interviewees for their time and insights. September 2005 © The Economist Intelligence Unit 2005 1 Quality manufacturing A blockbuster opportunity for pharmaceuticals Abstract Pharmaceutical manufacturing is shedding its “poor cousin” image and gaining in importance relative to R&D and marketing. Decades of regulatory and industry standard practice in manufacturing are being reworked to help push operating efficiency in pharma closer to other “normal” industries such as semiconductors, industrial chemicals and even consumer packaged goods. Early reports from the field suggest that progress will require massive change across organisations beyond the factory floor. However, the potential improvements in efficiency and regulatory oversight are too great to ignore. 2© The Economist Intelligence Unit 2005 Quality manufacturing A blockbuster opportunity for pharmaceuticals Introduction changing how it intends to approve pharmaceutical manufacturing applications by emphasising a “quality by design” model of regulatory approval compared with a “quality by test results” orientation that it used until recently. -

The Effect of Quality Management on the Service Quality and Business
International Journal of Quality & Reliability Management The effect of quality management on the service quality and business success of logistics service providers Wolfgang Kersten, Jan Koch, Article information: To cite this document: Wolfgang Kersten, Jan Koch, (2010) "The effect of quality management on the service quality and business success of logistics service providers", International Journal of Quality & Reliability Management, Vol. 27 Issue: 2, pp.185-200, https://doi.org/10.1108/02656711011014302 Permanent link to this document: https://doi.org/10.1108/02656711011014302 Downloaded on: 16 February 2018, At: 01:35 (PT) References: this document contains references to 55 other documents. To copy this document: [email protected] The fulltext of this document has been downloaded 6834 times since 2010* Users who downloaded this article also downloaded: (2010),"Service quality and its relation to satisfaction and loyalty in logistics outsourcing relationships", Managing Service Quality: An International Journal, Vol. 20 Iss 6 pp. 496-510 <a href="https:// doi.org/10.1108/09604521011092857">https://doi.org/10.1108/09604521011092857</a> (2008),"Logistics service quality: a new way to loyalty", Industrial Management & Data Systems, Vol. 108 Iss 5 pp. 650-668 <a href="https://doi.org/10.1108/02635570810876778">https:// doi.org/10.1108/02635570810876778</a> Access to this document was granted through an Emerald subscription provided by emerald-srm:438847 [] For Authors Downloaded by Hamburg University of Technology At 01:35 16 February 2018 (PT) If you would like to write for this, or any other Emerald publication, then please use our Emerald for Authors service information about how to choose which publication to write for and submission guidelines are available for all. -

Chapter 1 Introduction to Quality Assurance Review
Quality Assurance Review Handbook, 2012 Office of the Auditor General, Nepal Chapter 1 Introduction to Quality Assurance Review Purpose and Overview The purpose of this chapter is to emphasise the objective and importance of quality assurance (QA) in audit as it relates to the Office of the Auditor General, Nepal. The main purpose of this handbook is to provide a step-by-step approach for performing internal Quality Assurance Reviews. The chapter describes the basic concepts relating to quality, quality control and quality assurance. Distinctions of these concepts are achieved through the sections that explain the characteristics of quality and the differences between quality assurance and quality control. Quality Control (QC) and Quality Assurance (QA) are two different aspects of a robust quality control mechanism, and both are critical to the effectiveness of an SAI’s performance. Quality control is a term that encompasses the policies and procedures that are put in place in an OAGN to ensure that its audit work is of consistently high quality. Since the quality of OAGN products and services is of utmost importance in creating the desired impact on the external stakeholders, a Quality Assurance function needs to be established (or strengthened) in OAGN. The study of quality assurance is anchored on the related and appropriate quality standards and guidelines taken from the INTOSAI and ASOSAI which highlight the requirements for quality services and outputs of an SAI. This chapter concludes with a brief discussion on the types of QA reviews which can either be performed internally or by external persons, or may pertain to pre-issuance or post-audit reviews.