Synthesis, Properties and Uses of Chromium-Based Pigments from The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coulsonite Fev2o4—A Rare Vanadium Spinel Group Mineral in Metamorphosed Massive Sulfide Ores of the Kola Region, Russia
minerals Article Coulsonite FeV2O4—A Rare Vanadium Spinel Group Mineral in Metamorphosed Massive Sulfide Ores of the Kola Region, Russia Alena A. Kompanchenko Geological Institute of the Federal Research Centre “Kola Science Centre of the Russian Academy of Sciences”, 14 Fersman Street, 184209 Apatity, Russia; [email protected]; Tel.: +7-921-048-8782 Received: 24 August 2020; Accepted: 21 September 2020; Published: 24 September 2020 Abstract: This work presents new data on a rare vanadium spinel group mineral, i.e., coulsonite FeV2O4 established in massive sulfide ores of the Bragino occurrence in the Kola region, Russia. Coulsonite in massive sulfide ores of the Bragino occurrence is one of the most common vanadium minerals. Three varieties of coulsonite were established based on its chemical composition, some physical properties, and mineral association: coulsonite-I, coulsonite-II, and coulsonite-III. Coulsonite-I forms octahedral crystal clusters of up to 500 µm, and has a uniformly high content of 2 Cr2O3 (20–30 wt.%), ZnO (up to 4.5 wt.%), and MnO (2.8 wt.%), high microhardness (743 kg/mm ) and coefficient of reflection. Coulsonite-II was found in relics of quartz–albite veins in association with other vanadium minerals. Its features are a thin tabular shape and enrichment in TiO2 of up to 18 wt.%. Coulsonite-III is the most common variety in massive sulfide ores of the Bragino occurrence. Coulsonite-III forms octahedral crystals of up to 150 µm, crystal clusters, and intergrowths with V-bearing ilmenite, W-V-bearing rutile, Sc-V-bearing senaite, etc. Chemical composition of coulsonite-III is characterized by wide variation of the major compounds—Fe, V, Cr. -

Chemistry, Geochemistry, and Geology of Chromium and Chromium Compounds
L1608_C02.fm Page 23 Thursday, July 15, 2004 6:57 PM 2 Chemistry, Geochemistry, and Geology of Chromium and Chromium Compounds William E. Motzer and Todd Engineers CONTENTS 2.1 Chromium Chemistry .................................................................................24 2.1.1 Background ......................................................................................24 2.1.2 Elemental/Metallic Chromium Characteristics .........................25 2.1.3 Ionic Radii ........................................................................................29 2.1.4 Oxidation States...............................................................................30 2.1.5 Stable and Radioactive Isotopes ...................................................31 2.1.6 Characteristics of Chromium Compounds.................................34 2.2 Natural Chromium Concentrations..........................................................34 2.2.1 Mantle ...............................................................................................46 2.2.2 Chromium Minerals........................................................................46 2.2.3 Chromium Ore Deposits................................................................46 2.2.3.1 Stratiform Mafic-Ultramafic Chromite Deposits .........62 2.2.3.2 Podiform- or Alpine-Type Chromite Deposits ............63 2.2.4 Crude Oil, Tars and Pitch, Asphalts, and Coal..........................63 2.2.5 Rock ...................................................................................................64 -

Malayaite Casnsio5 C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Monoclinic
Malayaite CaSnSiO5 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Monoclinic. Point Group: 2=m: Crystals wedge-shaped, up to 3 cm; massive, as a coating on cassiterite. Physical Properties: Hardness = 3.5{4 D(meas.) = 4.3(2) D(calc.) = [4.55] Fluoresces pale to bright yellow or yellow-green under UV. Optical Properties: Translucent. Color: Very pale creamy yellow to deep yellow-orange, colorless. Luster: Resinous. Optical Class: Biaxial ({). ® = 1.764{1.765 ¯ = 1.783{1.786 ° = 1.798{1.801 2V(meas.) = 84±{86± Cell Data: Space Group: A2=a: a = 7.156(6) b = 8.895(9) c = 6.668(4) ¯ = 113:4(1)± Z = 4 X-ray Powder Pattern: Synthetic. 3.28 (100), 5.05 (50), 2.639 (45), 3.06 (35), 2.665 (30), 2.412 (20), 2.099 (20) Chemistry: (1) (2) SiO2 21.26 22.49 SnO2 58.48 56.38 FeO 0.15 CaO 19.14 20.94 LOI 0.50 Total 99.38 99.96 (1) Sungei Lah Valley, Malaya; corresponds to Ca0:95Sn1:07Si0:98O5: (2) Gumble, Australia; by electron microprobe, corresponding to Ca1:00Sn1:00Fe0:01Si1:00O5: Occurrence: In tin-rich contact metamorphic skarn deposits, probably a hydrothermal alteration product of cassiterite or other tin-bearing minerals. Association: Cassiterite, quartz, calcite, wollastonite, pyroxene, garnet. Distribution: In the Sungei Lah Valley, Chenderiang, Perak State, and other locations in Malaya, Malaysia. From Piniok, Thailand. In the Toroku and Mitate mines, Miyazaki Prefecture; the Sampo mine, Okayama Prefecture; the Hoei mine, Oita Prefecture; and the Kuga mine, Yamaguchi Prefecture, Japan. -

O, a New Mineral of the Titanite Group from the Piława Górna Pegmatite, the Góry Sowie Block, Southwestern Poland
Mineralogical Magazine, June 2017, Vol. 81(3), pp. 591–610 Żabińskiite, ideally Ca(Al0.5Ta 0.5)(SiO4)O, a new mineral of the titanite group from the Piława Górna pegmatite, the Góry Sowie Block, southwestern Poland 1,* 2 3 3 4 ADAM PIECZKA ,FRANK C. HAWTHORNE ,CHI MA ,GEORGE R. ROSSMAN ,ELIGIUSZ SZEŁĘG , 5 5 6 6 7 ADAM SZUSZKIEWICZ ,KRZYSZTOF TURNIAK ,KRZYSZTOF NEJBERT ,SŁAWOMIR S. ILNICKI ,PHILIPPE BUFFAT AND 7 BOGDAN RUTKOWSKI 1 AGH University of Science and Technology, Department of Mineralogy, Petrography and Geochemistry, 30-059 Kraków, Mickiewicza 30, Poland 2 Department of Geological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada 3 Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, 91125-2500, California, USA 4 University of Silesia, Faculty of Earth Sciences, Department of Geochemistry, Mineralogy and Petrography, 41-200 Sosnowiec, Bedzin̨ ská 60, Poland 5 University of Wrocław, Institute of Geological Sciences, 50-204 Wrocław, M. Borna 9, Poland 6 University of Warsaw, Faculty of Geology, Institute of Geochemistry, Mineralogy and Petrology, 02-089 Warszawa, Żwirki and Wigury 93, Poland 7 AGH University of Science and Technology, International Centre of Electron Microscopy for Materials Science, Department of Physical and Powder Metallurgy, 30-059 Kraków, Mickiewicza 30, Poland [Received 7 January 2016; Accepted 21 April 2016; Associate Editor: Ed Grew] ABSTRACT Ż ́ ł abinskiite, ideally Ca(Al0.5Ta0.5)(SiO4)O, was found in a Variscan granitic pegmatite at Pi awa Górna, Lower Silesia, SW Poland. The mineral occurs along with (Al,Ta,Nb)- and (Al,F)-bearing titanites, a pyrochlore-supergroup mineral and a K-mica in compositionally inhomogeneous aggregates, ∼120 μm× 70 μm in size, in a fractured crystal of zircon intergrown with polycrase-(Y) and euxenite-(Y). -

Stabilization of Transition Metal Chromite Nanoparticles in Silica
World Academy of Science, Engineering and Technology International Journal of Chemical and Molecular Engineering Vol:8, No:11, 2014 6WDELOL]DWLRQ RI 7UDQVLWLRQ 0HWDO &KURPLWH1DQRSDUWLFOHV LQ 6LOLFD 0DWUL[ Jiri Plocek, Petr Holec, Simona Kubickova, Barbara Pacakova, Irena Matulkova, Alice Mantlikova, Ivan Nemec, Daniel NiznanskyJana Vejpravova Abstract—This article presents summary on preparation and temperature. The magnetic ordering is therefore characteristic characterization of zinc, copper, cadmium and cobalt chromite by a considerable spin frustration and strongly depend on nanocrystals, embedded in an amorphous silica matrix. The the chemical order (the spinel inversion, oxygen deficit etc.) ZnCr2O4/SiO2, CuCr2O4/SiO2, CdCr2O4/SiO2 and CoCr2O4/SiO2 nanocomposites were prepared by a conventional sol-gel method and on the cation site occupancy in the spinel structure [8] under acid catalysis. Final heat treatment of the samples was carried (diamagnetic, paramagnetic or JT active), respectively. ◦ out at temperatures in the range of 900 − 1200 C to adjust the The zinc chromite is known as a frustrated antiferromagnet phase composition and the crystallite size, respectively. The resulting with a complex coplanar spin structure below the Neel´ samples were characterized by Powder X-ray diffraction (PXRD), temperature, T = 12 K [9] and it is arguably the most High Resolution Transmission Electron Microscopy (HRTEM), N Raman/FTIR spectroscopy and magnetic measurements. Formation magnetically-frustrated system known so far. At room 3+ of the spinel phase was confirmed in all samples. The average size of temperature, it has a cubic crystal structure where Cr the nanocrystals was determined from the PXRD data and by direct ions form a network of pyrochlore-like lattice [10]. -

Structural Investigations Along the Join Catiosio4-Casnosio4
Structural investigations along the join CaTiOSiO4-CaSnOSiO4 Autor(en): Kunz, Martin / Xirouchakis, Dimitros / Wang, Yanbin Objekttyp: Article Zeitschrift: Schweizerische mineralogische und petrographische Mitteilungen = Bulletin suisse de minéralogie et pétrographie Band (Jahr): 77 (1997) Heft 1 PDF erstellt am: 05.10.2021 Persistenter Link: http://doi.org/10.5169/seals-58464 Nutzungsbedingungen Die ETH-Bibliothek ist Anbieterin der digitalisierten Zeitschriften. Sie besitzt keine Urheberrechte an den Inhalten der Zeitschriften. Die Rechte liegen in der Regel bei den Herausgebern. Die auf der Plattform e-periodica veröffentlichten Dokumente stehen für nicht-kommerzielle Zwecke in Lehre und Forschung sowie für die private Nutzung frei zur Verfügung. Einzelne Dateien oder Ausdrucke aus diesem Angebot können zusammen mit diesen Nutzungsbedingungen und den korrekten Herkunftsbezeichnungen weitergegeben werden. Das Veröffentlichen von Bildern in Print- und Online-Publikationen ist nur mit vorheriger Genehmigung der Rechteinhaber erlaubt. Die systematische Speicherung von Teilen des elektronischen Angebots auf anderen Servern bedarf ebenfalls des schriftlichen Einverständnisses der Rechteinhaber. Haftungsausschluss Alle Angaben erfolgen ohne Gewähr für Vollständigkeit oder Richtigkeit. Es wird keine Haftung übernommen für Schäden durch die Verwendung von Informationen aus diesem Online-Angebot oder durch das Fehlen von Informationen. Dies gilt auch für Inhalte Dritter, die über dieses Angebot zugänglich sind. Ein Dienst der ETH-Bibliothek ETH Zürich, Rämistrasse 101, 8092 Zürich, Schweiz, www.library.ethz.ch http://www.e-periodica.ch SCHWEIZ. MINERAL. PETROGR. MITE 77, 1-11,1997 Structural investigations along the join CaTi0Si04-CaSn0Si04 by Martin Kunz', Dimitrios Xirouchakis2, Yanbin Wang3, John B. Parise2 and Donald H. Lindsley2 Abstract High resolution synchrotron X-ray data were used to structurally characterize a series of compounds along the sol- id-solution titanite (HT-phase)-malayaite. -

Mineral Ecology and Network Analysis of Chromium, Platinum
MINERAL ECOLOGY AND NETWORK ANALYSIS OF CHROMIUM, PLATINUM, GOLD AND PALLADIUM A THESIS IN ENVIRONMENTAL AND URBAN GEOSCIENCES Presented to the Faculty of the University Of Missouri-Kansas City in partial fulfillment of The requirements for the degree MASTER OF SCIENCE By CHARLES ANDENGENIE MWAIPOPO B.S., University of Missouri-Kansas City, 2018 Kansas City, Missouri 2020 MINERAL ECOLOGY AND NETWORK ANALYSIS OF CHROMIUM, PLATINUM, GOLD AND PALLADIUM Charles Andengenie Mwaipopo, Candidate for the Master of Science Degree University of Missouri-Kansas City, 2020 ABSTRACT Data collected on the location of mineral species and related minerals from the field have many great uses from mineral exploration to mineral analysis. Such data is useful for further exploration and discovery of other minerals as well as exploring relationships that were not as obvious even to a trained mineralogist. Two fields of mineral analysis are examined in the paper, namely mineral ecology and mineral network analysis through mineral co-existence. Mineral ecology explores spatial distribution and diversity of the earth’s minerals. Mineral network analysis uses mathematical functions to visualize and graph mineral relationships. Several functions such as the finite Zipf-Mandelbrot (fZM), chord diagrams and mineral network diagrams, processed data and provided information on the estimation of minerals at different localities and interrelationships between chromium, platinum, gold and palladium-bearing minerals. The results obtained are important in highlighting several connections that could prove useful in mineral exploration. The main objective of the study is to provide any insight into the relationship among chromium, platinum, palladium and gold that could prove useful in mapping out potential locations of either mineral in the future. -

Titanite (Sphene) Outline Crystal Structure of Titanite
Titanite (Sphene) CaTiO(SiO4) By Dominic Papineau and Tiffany Yesavage Titanite refers to titanium which was named after the Titans, the mythical first sons of the Earth. Outline 1 Chemical composition 2 Physical properties 3 Optical properties 4 Crystal structure 5 Titanite stability 6 Occurrences 7 Uses Crystal structure of titanite Ca polyhedron Ti octahedron Si tetrahedron O atoms 1 Chemical substitutions In Titanium octahedra: Most commonly Al3+ or Fe3+ replaces Ti4+. Occasionally, Fe2+ and REE such as Nb5+ and Ta5+ may also substitute for Ti4+ . For Oxygen: O2- is commonly replaced by either F- or OH-. The most common substitution that occurs in titanite involves this coupled substitution: (Al,Fe)3+ + O2- >> Ti4+ + (OH-, F -) Notice that the above charges balance. This particular substitution may occur in up to 30% of cations in titanite. Chemical substitutions (continued) For Calcium: Sr, Ba, Na, Mn and REE such as Ce and Nd may substitute for Ca2+ . U, Th and radiogenic Pb may also substitute for Calcium. Like zircon and apatite, titanite may be used in order to determine the ages of rocks. In the Silica tetrahedra: The only element observed to substitute for Si4+ is Al3+ . A complete solid solution has also been shown to exist between o CaTiO(SiO4) and SnTiO(SiO4) at 700 C and 7kbar. SnTiO(SiO4) is the chemical composition of malayaite. Malayaite and the high temperature form of titanite are they are isostructural. Physical properties Color: gray, brown, green, yellow, black, transparent to translucent Luster: resinous to adamantine Cleavage: {110} distinct Hardness: 5 - 5.5 Because of titanite’s lack of hardness, it weathers quickly in most rocks Specific gravity: 3.4 - 3.55 2 Optical properties Titanite under thin section can be determined from its “sphenoidal shape” as seen in a thin section. -
Linking Crystal Chemistry and Physical Properties of Natural and Synthetic Spinels: an UV-VIS-NIR and Raman Study
Dottorato di ricerca in Scienze della Terra Ciclo XXVI Linking crystal chemistry and physical properties of natural and synthetic spinels: an UV-VIS-NIR and Raman study Settore Scientifico Disciplinare GEO/06 Candidato Docente guida: Veronica D’Ippolito Prof. Giovanni B. Andreozzi Anni 2010-2013 “La mineralogia è una scienza di elite, ha pochi sbocchi professionali. Ma, come in tutte le cose della vita, se ci metti passione e determinazione, una strada vedrai che te la crei” Prof. Sergio Lucchesi “Success is getting what you want. Happiness is liking what you get.” H. Jackson Brown Index Introduction………………………………………………………………....... 1 Chapter 1- The Spinel Group……………………….…………………... 5 1.1- Crystal chemistry……………………………………………………………. 5 1.2- Structure……………………………………………………………………... 8 1.3- Relevance of spinels in the geological field……………………………… 16 1.4- Relevance of spinels in the gemological field …………………………… 19 1.5- Relevance of spinels in the technological field …...…………………….. 20 Chapter 2- Spectroscopic methods…………….……………………. 22 2.1- Introduction to spectroscopy………………………………………………. 22 2.2- Raman spectroscopy...…………………………………………………….. 25 2.2.1- Symmetry, Group theory, Normal Modes and Selection rules … 30 2.2.2- Applications………………………………………………….……….. 33 2.2.3- Raman spectra of Spinel Group compounds…………………….. 36 2.2.4- Luminescence spectroscopy using Raman spectrometer…….… 40 2.3- Optical absorption spectroscopy………………………………………….. 42 2.3.1- Crystal Field Theory (CFT)…………………………….…………… 45 One-electron systems…………………………….……………... 45 Crystal field splitting…………………………….……………….. 47 Many-electron dN systems…………………………………….… 50 Tanabe-Sugano diagrams………………………….…………… 50 2.3.2- Qualitative measurements in optical absorption spectra……….. 52 Causes of color………………………….……………………….. 53 2.3.3- Quantitative analysis in optical absorption spectra……………… 56 Structural relaxation……………………………………………... 57 i Chapter 3- Materials and methods………………………………....... 60 3.1- Materials………………………………………………………………….…. -
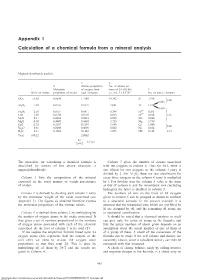
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

Malayaite Ceramic Pigments: a Combined Optical Spectroscopy and Neutron/X-Ray Diffraction Study
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PUblication MAnagement Published on the Materials Research Bulletin, 44 (2009) 1778-1785. Copyright © 2009 Elsevier Ltd. All rights reserved. doi: 10.1016/j.materresbull.2009.03.006 Malayaite Ceramic Pigments: a Combined Optical Spectroscopy and Neutron/X-ray Diffraction Study Giuseppe Cruciani1, Michele Dondi2, Matteo Ardit1, Teodora Stoyanova Lyubenova3, Juan B. Carda3, Francesco Matteucci2, Anna L. Costa2 1Department of Earth Sciences, University of Ferrara, Via Saragat 1, 44100 Ferrara, Italy 2ISTEC-CNR, Institute of Science and Technology for Ceramics, Via Granarolo 64, 48018 Faenza, Italy 3Dept of Inorganic and Organic Chemistry, University Jaime I, Campus Riu Sec, 12071 Castellón, Spain Abstract. Ceramic pigments based on the Cr-doped malayaite structure were synthesized by solid state reaction and characterized by optical spectroscopy and combined X-ray and neutron powder diffraction in order to elucidate the still unclear chromium substitution mechanisms. The results show that coloration is actually due to simultaneous occurrence of Cr4+ and Cr3+ ions in the crystal lattice. Spectroscopy data confirm that Cr4+ is replacing Sn4+ in the octahedral site and, in minor amount, Si4+ in the tetrahedral site. In addition, neutron powder diffraction data suggest that Cr3+ substitution for octahedral Sn4+ is charge balanced by formation of oxygen vacancies with no preference over the different oxygen sites. Upon incorporation of Cr ion, the SnO6 octahedra exhibit an off-centre displacement of central cation which in turn induces a rearrangement of both the octahedral and tetrahedral coordination shells. Key-words: ceramic pigment, crystal structure, malayaite, neutron diffraction, optical spectroscopy, X-ray diffraction. -
![Arxiv:1204.3007V2 [Cond-Mat.Mtrl-Sci] 23 Oct 2012 Thermal Changes in Molecular Conformation; And, Third, the High-Pressure Form of The](https://docslib.b-cdn.net/cover/3360/arxiv-1204-3007v2-cond-mat-mtrl-sci-23-oct-2012-thermal-changes-in-molecular-conformation-and-third-the-high-pressure-form-of-the-7363360.webp)
Arxiv:1204.3007V2 [Cond-Mat.Mtrl-Sci] 23 Oct 2012 Thermal Changes in Molecular Conformation; And, Third, the High-Pressure Form of The
1 PASCal: A principal-axis strain calculator for thermal expansion and compressibility determination Matthew J. Cliffe and Andrew L. Goodwin * Department of Chemistry, University of Oxford, Inorganic Chemistry Laboratory, South Parks Road, Oxford, OX1 3QR U.K.. E-mail: [email protected] (Received 0 XXXXXXX 0000; accepted 0 XXXXXXX 0000) Abstract We describe a web-based tool (pascal; principal axis strain calculator, http://pascal.chem.ox.ac.uk) designed to simplify the determination of principal coefficients of thermal expansion and compressibilities from variable-temperature and variable-pressure lattice param- eter data. In a series of three case studies, we use pascal to re-analyse previously- published lattice parameter data and show that additional scientific insight is obtain- able in each case. First, the two-dimensional metal{organic framework Cu-SIP-3 is found to exhibit the strongest area-negative thermal expansion (NTE) effect yet observed; second, the widely-used explosive HMX exhibits much stronger mechanical anisotropy than had previously been anticipated, including uniaxial NTE driven by arXiv:1204.3007v2 [cond-mat.mtrl-sci] 23 Oct 2012 thermal changes in molecular conformation; and, third, the high-pressure form of the mineral malayaite is shown to exhibit a strong negative linear compressibility (NLC) effect that arises from correlated tilting of SnO6 and SiO4 coordination polyhedra. PREPRINT: Journal of Applied Crystallography A Journal of the International Union of Crystallography 2 1. Introduction The mechanisms by which crystalline materials respond to changes in temperature and pressure are as important for the valuable insight they provide into the funda- mental chemistry of the solid state as they are for a range of practical applications.