Myeloma Cells with Asurophilic Granules - an Unusual Morphological Variant – Case Presentation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cytology of Myeloma Cells
J Clin Pathol: first published as 10.1136/jcp.29.10.916 on 1 October 1976. Downloaded from J. clin. Path., 1976, 29, 916-922 Cytology of myeloma cells F. G. J. HAYHOE AND ZOFIA NEUMAN1 From the Department of Haematological Medicine, Cambridge University SYNOPSIS A cytological, cytochemical, and cytometric study of plasma cells from 195 cases of multiple myeloma showed that, contrary to earlier reports, flaming cells, thesaurocytes, and intra- nuclear inclusions are not confined to IgA-secreting cases but are common also in IgG and Bence Jones varieties of myeloma. IgA-secreting cells are not larger, nor do they have a lower nuclear- cytoplasmic ratio than other myeloma cells. On average, for a given mass of tumour, Bence-Jones, IgG, and IgA varieties of myeloma produce amounts of paraprotein in the ratio 1 to 1 6 to 2-7. In 1961 Paraskevas et al reported a correlation the results of a larger scale survey carried out some between the morphological features of plasma cells years ago but previously unpublished. in myeloma and the type of immunoglobulin secreted. The cases studied included 12 with y1A Material and methods (f2A, IgA) myeloma, 30 with y (IgG) myeloma, and six myelomas without M protein (probably Bence The study was performed on bone marrow smearscopyright. Jones myelomas). Flaming cells, thesaurocytes, and from 200 consecutive patients newly entered into a intranuclear, PAS-positive inclusion bodies were comparative trial of treatments in myeloma, under found only in cases of IgA myeloma, and flaming the auspices of the Medical Research Council. Five cells especially were present in most cases and in patients were subsequently excluded as not confirmed high percentage in several. -

Cytology of Inflammation
Association of Avian Veterinarians Australasian Committee Ltd. Annual Conference Proceedings Auckland New Zealand 2017 25: 20-30 Cytology of Inflammation Terry W. Campbell MS, DVM, PhD, Emeritus Department of Clinical Sciences College of Veterinary Medicine and Biomedical Sciences Colorado State University 300 West Drake Road Fort Collins, Colorado, USA The inflammatory response of birds can be classified as a mixed cell inflammation, the most common cellular in- either heterophilic, eosinophilic (rarely reported as they flammatory response seen in birds. They can develop into may be difficult to detect with routine staining), mixed epithelioid and multinucleated giant cells. As the inflam- cell, or macrophagic (histiocytic) depending upon the pre- matory process continues and becomes chronic, granu- dominant cell type. Inflammatory cells arrive at the lesion lomas may develop as the macrophages form into layers by active migration in response to various chemotactic that resemble epithelium and this is the reason for the factors, and the type of inflammatory response present term “epithelioid cells.” As the lesion matures, fibroblasts may suggest a possible aetiology and pathogenesis. proliferate and begin to lay down collagen. These prolif- erating fibroblasts appear large compared to the small Heterophilic Inflammation of Birds densely staining fibroblasts of normal fibrous tissue. Lym- phocytes appear within the stroma and participate in the Inflammation occurs whenever chemotactic factors for cell-mediated immune response. Fusion of macrophages inflammatory cells are released. The most common caus- into giant cells occurs in association with material that is es are microbes and their toxins, physical and chemical not readily digested by macrophages. The results of acute trauma, death of cells from circulatory insufficiency, and inflammation may be complete resolution, development immune reactions. -

Reptile Clinical Pathology Vickie Joseph, DVM, DABVP (Avian)
Reptile Clinical Pathology Vickie Joseph, DVM, DABVP (Avian) Session #121 Affiliation: From the Bird & Pet Clinic of Roseville, 3985 Foothills Blvd. Roseville, CA 95747, USA and IDEXX Laboratories, 2825 KOVR Drive, West Sacramento, CA 95605, USA. Abstract: Hematology and chemistry values of the reptile may be influenced by extrinsic and intrinsic factors. Proper processing of the blood sample is imperative to preserve cell morphology and limit sample artifacts. Identifying the abnormal changes in the hemogram and biochemistries associated with anemia, hemoparasites, septicemias and neoplastic disorders will aid in the prognostic and therapeutic decisions. Introduction Evaluating the reptile hemogram is challenging. Extrinsic factors (season, temperature, habitat, diet, disease, stress, venipuncture site) and intrinsic factors (species, gender, age, physiologic status) will affect the hemogram numbers, distribution of the leukocytes and the reptile’s response to disease. Certain procedures should be ad- hered to when drawing and processing the blood sample to preserve cell morphology and limit sample artifact. The goal of this paper is to briefly review reptile red blood cell and white blood cell identification, normal cell morphology and terminology. A detailed explanation of abnormal changes seen in the hemogram and biochem- istries in response to anemia, hemoparasites, septicemias and neoplasia will be addressed. Hematology and Chemistries Blood collection and preparation Although it is not the scope of this paper to address sites of blood collection and sample preparation, a few im- portant points need to be explained. For best results to preserve cell morphology and decrease sample artifacts, hematologic testing should be performed as soon as possible following blood collection. -

Intranuclear Inclusions in Myeloma Patient
Published online: 2021-05-24 Letter to Editor Pathologist’s Feast: Intranuclear Inclusions in Myeloma Patient Sir, aspiration [Figure 2]. Plasma cells showed Dutcher body We present a case of a 36‑year‑old female admitted in bone marrow aspiration [Figure 3] as periodic acid– in hospital with complaints of pain in sacral region Schiff positive intranuclear inclusion [Figure 4]. The bone radiating toward right lower limb for 1 month. Laboratory marrow biopsy showed loss of normal architecture with examination revealed hemoglobin 8.1 g/dL, red blood packed marrow studded by plasma cells [Figure 5]. cell count – 2.61 × 109/mm3, white blood cell count Multiple myeloma account for 1% of all cancers and 16.16 × 103/mm3, and platelet count 299 × 109/mm3. The approximately 10% of all hematological malignancies.[1] The differential showed polymorphs – 74%, lymphocytes – 22%, eosinophils – 1%, and monocytes – 3%. Peripheral blood peak incidence is seventh decade, and it is quite rare, below smear showed rouleaux formation in red blood cells. The 40 years of age. The clinical and biological characteristics serum biochemistry showed blood urea – 54 mg/dl and of multiple myeloma in young patients are similar to those [2] creatinine – 3.8 mg/dl, angiotensin converting enzyme in elderly as in literature in studies by Usha et al. and [3] level – 64.25 U/L, and serum calcium – 13.3 mg/dl. Liver Bladé et al. The above case shows ditcher body inclusions function tests and serum electrolytes were normal and in plasma cells on bone marrow aspiration. HIV and HBsAg were nonreactive. -

Hyperviscosity Syndrome Complicating Rheumatoid Arthritis: Report of Two Additional Cases and Review of the Literature
Henry Ford Hospital Medical Journal Volume 30 Number 4 Article 3 12-1982 Hyperviscosity Syndrome Complicating Rheumatoid Arthritis: Report of Two Additional Cases and Review of the Literature Michael R. Lovy Mark C. Kranc Gilbert B. Bluhm Jeanne M. Riddle Paul D. Stein Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Lovy, Michael R.; Kranc, Mark C.; Bluhm, Gilbert B.; Riddle, Jeanne M.; and Stein, Paul D. (1982) "Hyperviscosity Syndrome Complicating Rheumatoid Arthritis: Report of Two Additional Cases and Review of the Literature," Henry Ford Hospital Medical Journal : Vol. 30 : No. 4 , 189-198. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol30/iss4/3 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp Med J Vol 30, No 4,1982 Clinical Reports Hyperviscosity Syndrome Complicating Rheumatoid Arthritis: Report of Two Additional Cases and Review of the Literature Michael R. Lovy, MD,* Mark C. Krane, MD,** Gilbert B. Bluhm, MD, *** Jeanne M. Riddle, PhD,*** and Paul D. Stein, MD**** A hyperviscosity syndrome developed in two patients ity caused by the presence ofthe high molecular weight with rheumatoid arthritis. Analytic ultracentrifugation complexes. These abnormalities, as well as the clinical of the sera from one patient demonstrated 225 com bleeding, whole blood, and plasma viscosity, became plexes. Intermediate, 22S, and 375 complexes were normal after treatment. -

Plasma Cell Myeloma, Plasmacytoma
Plasma Cell Neoplasms Plasma cell neoplasms: definition • Immunosecretory disorders result from the expansion of a single clone of immunoglobulin secreting, terminally differentiated, end-stage B- cells. • These monoclonal proliferations of either plasma cells or plasmocytoid lymphocytes are characterised by secretion of a single homogeneous immunoglobulin product known as the M-component or monoclonal component. Plasma cell neoplasms: definition • The prominence of the M-component in serum and urine protein electrophoresis (SPE, UPE) has led to various designations for these disorders including monoclonal gammopathies, dysproteinemias and paraproteinemias. • The M-components, although monoclonal, may be seen in both malignant conditions (plasma cell myeloma and Waldenström macroglobulinemia) and benign or premalignant disorders (MGUS). Plasma cell neoplasms: definition • Among these gammopathies are a number of clinicopathological entities, some being primarily plasmacytic, including plasma cell (multiple) myeloma and plasmacytoma; while others contain also lymphocytes, including the heavy chain diseases and Waldenström macroglobulinemia. Plasma cell neoplasms: definition • Variants of plasma cell myeloma include syndromes defined by the consequence of tissue immunoglobulin deposition, including (1) primary amyloidosis (AL), and (2) light and heavy chain deposition diseases. Plasma Cell Myeloma Plasma Cell Myeloma: Definition • Bone marrow based, multifocal plasma cell neoplasm characterised by a serum monoclonal protein and skeletal destruction with osteolytic lesions, pathological fractures, bone pain, hypercalcemia, and anemia. • The disease spans a spectrum from localized, smoldering or indolent to aggressive, disseminated forms with plasma cell infiltration of various organs, plasma cell leukemia and deposition of abnormal Ig chains in tissues. Plasma Cell Myeloma: Definition • The diagnosis is based on a combination of pathological, radiological, and clinical features. -

GG Pur Bone Marrow Fm/C1 FNL-Br
$50. 00 Bone Marrow Evaluation in Dogs and Cats Maxey L. Wellman, DVM, PhD, DACVP M. Judith Radin, DVM, PhD, DACVP Clinical Handbook Series Published by The Gloyd Group, Inc. Wilmington, Delaware © 2004 by Nestlé Purina PetCare Company. All rights reserved. Printed in the United States of America. Nestlé Purina PetCare Company: Checkerboard Square, Saint Louis, Missouri, 63188 First printing, 1999. This book is protected by copyright. ISBN 0-9678005-0-1 Table of Contents Introduction ......................................1 Part I Chapter 1 Normal Hematopoiesis ......................................5 Chapter 2 Indications for Bone Marrow Evaluation ................................13 Chapter 3 Procedures for Bone Marrow Aspiration and Biopsy ........................................................15 Chapter 4 Cytologic and Histologic Evaluation of the Bone Marrow Bone Marrow ....................................................17 Evaluation Part II in Dogs and Cats Chapter 5 Evaluation of Abnormal Bone Marrow ..........29 Maxey L. Wellman, DVM, PhD, DACVP Chapter 6 M. Judith Radin, DVM, PhD, DACVP Hematopoietic Neoplasia .................................43 Chapter 7 Case Studies .....................................................61 Part III Hematology Reference Ranges of the Dog and Cat .....................................................89 Index of Figures ...............................................90 Glossary of Terms ............................................96 Suggested Reading .........................................100 Clinical -
Hematolymphoid-System.Pdf
Toxicologic Pathology 2019, Vol. 47(6) 665-783 ª The Author(s) 2019 Nonproliferative and Proliferative Article reuse guidelines: sagepub.com/journals-permissions Lesions of the Rat and Mouse DOI: 10.1177/0192623319867053 journals.sagepub.com/home/tpx Hematolymphoid System 1 2,c Cynthia L. Willard-Mack, (Chair) , Susan A. Elmore , 3 4,e,g 5,f William C. Hall , Johannes Harleman , C. Frieke Kuper , 6,a 7,d,g 8,g Patricia Losco , Jerold E. Rehg , Christine Ru¨ hl-Fehlert , 9,d,g 10,b 11 Jerrold M. Ward , Daniel Weinstock , Alys Bradley , 12 13 14 Satoru Hosokawa , Gail Pearse , Beth W. Mahler , 2 15 Ronald A. Herbert , and Charlotte M. Keenan Abstract The INHAND Project (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) is a joint initiative of the Societies of Toxicologic Pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP), and North America (STP) to develop an internationally accepted nomenclature for proliferative and nonproliferative changes in rats and mice. The purpose of this publication is to provide a standardized nomenclature for classifying changes observed in the hematolymphoid organs, including the bone marrow, thymus, spleen, lymph nodes, mucosa-associated lymphoid tissues, and other lymphoid tissues (serosa-associated lymphoid clusters and tertiary lymphoid structures) with color photomicrographs illustrating examples of the lesions. Sources of material included histopathology databases from government, academia, and industrial laboratories throughout the world. Content includes spontaneous lesions as well as lesions induced by exposure to test materials. The nomenclature for these organs is divided into 3 terminologies: descriptive, conventional, and enhanced. -
Plasma Cell Neoplasms 1
4/15/17 Learning Objectives Plasma Cell Neoplasms 1. Define monoclonal gammopathy 2. Compare and contrast the clinical and Review and Case Studies laboratory features of the following plasma cell disorders: Plasmacytoma, Asymptomatic and Symptomatic plasma cell myeloma and Monoclonal gammopathy of undetermined significance (MGUS). John H. Landis, MS, MT(ASCP) 3. Differentiate lymphoplasmacytic lymphoma University of Cincinnati from plasma cell myeloma Oakland University Professor Emeritus: Ferris State University 2 Introduction Normal Plasma Cell • Plasma Cell Myeloma (PCM) (multiple myeloma, myelomatosis, medullary plasmacytosis) • B-cell neoplasm resulting from expansion of a single clone of plasma cells (PCs) • Abnormal PCs synthesize monoclonal immunoglobulin (M protein), a monoclonal light chain (Bence Jones protein) or both. 3 4 Normal Plasma Cell Introduction Nuclear Cistern • Normal Plasma Cells – About 1% of the normal marrow cells – Ovoid cells with an ovoid nucleus set at 90o to cell Golgi orientation (hof) – Blue cytoplasm due to increase in ribosomal RNA in the RER making protein - immunoglobulin Lysosome RER 5 6 1 4/15/17 Introduction Introduction • Protein factories producing immunoglobulin PCM is clinically heterogeneous – from no composed of Light chains (kappa and lambda) symptoms to aggressive disease and Heavy chains (G, A, M, D or E) • Usually confined to BM with extramedullary involvement only in end stage disease • Represent 10-15% of all hematologic malignancies and 1% of all malignancies • Median age at diagnosis -
A Laboratory Guide to Clinical Hematology
A Laboratory Guide to Clinical Hematology A Laboratory Guide to Clinical Hematology A Laboratory Guide to Clinical Hematology VALENTIN VILLATORO AND MICHELLE TO EDMONTON A Laboratory Guide to Clinical Hematology by Michelle To is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, except where otherwise noted. Please be aware that the content for the entirety of this eBook is subject to a creative common license: Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) You are free to: Share — copy and redistribute the material in any medium or format Adapt — remix, transform, and build upon the material The licensor cannot revoke these freedoms as long as you follow the license terms. Under the following terms: Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. NonCommercial — You may not use the material for commercial purposes. No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits. Contents Authors & Editors ................................................................................................................................... xii Creative Commons License and Citation ............................................................................................... xiii Contact Information and Feedback ........................................................................................................ -

Histopathological Bodies in Oral Pathology 1NJ Naziya, 2P Jayanthi, 3RK Harish, 4R Rathy, 5S Sunil
OMPJ NJ Naziya et al 10.5005/jp-journals-10037-1114 MINI REVIEW Histopathological Bodies in Oral Pathology 1NJ Naziya, 2P Jayanthi, 3RK Harish, 4R Rathy, 5S Sunil ABSTRACT (A) Infectious diseases: Introduction: Histopathological bodies refer to the cell bodies • Henderson–Peterson bodies that are associated with the name of the scientist who first • Cowdry type I bodies described them. The histopathological bodies are either intra • Cowdry type II bodies cellular or extracellular abnormal bodies seen specifically • Negri bodies in certain diseases. These structures appear within the cell • Toto bodies nucleus, the cytoplasm, or in both. (B) Neoplasms: Objectives: This article lists some of the named cell bodies • Wagner–Meissner bodies seen in routine histopathological practice with a brief description • Verocay bodies about their morphology and staining reactions. • Russell bodies Conclusion: Histopathological bodies represent peculiar mor • Pustulo-ovoid bodies of Milian phological alterations in a tissue giving rise to a highly specific pattern. The presence of these bodies is often an important • Kamino bodies diagnostic aid in identifying the underlying disease. • Dutcher bodies (C) Autoimmune diseases: Keywords: Cell bodies, Histopathology, Morphology. • Civatte bodies How to cite this article: Naziya NJ, Jayanthi P, Harish RK, • Schaumann bodies Rathy R, Sunil S. Histopathological Bodies in Oral Pathology. Oral Maxillofac Pathol J 2017;8(2):114-117. (D) Blood dyscrasias: • Heinz bodies Source of support: Nil • Howell–Jolly bodies Conflict of interest: None • Fessas bodies (E) Inflammatory lesions: INTRODUCTION • Rushton bodies Histopathological bodies are either intracellular or Histopathological Bodies seen in Physiological extracellular abnormal bodies seen specifically in certain Conditions diseases. These structures appear within the cell nucleus or the cytoplasm or in both, and exhibit characteristic Odland Bodies (Lamellar Body) staining properties. -
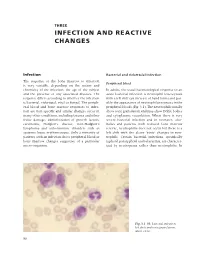
Infection and Reactive Changes
THREE INFECTION AND REACTIVE CHANGES Infection Bacterial and rickettsial infection The response of the bone marrow to infection Peripheral blood is very variable, depending on the nature and chronicity of the infection, the age of the subject In adults, the usual haematological response to an and the presence of any associated diseases. The acute bacterial infection is neutrophil leucocytosis response differs according to whether the infection with a left shift (an increase of band forms and pos- is bacterial, rickettsial, viral or fungal. The periph- sibly the appearance of neutrophil precursors in the eral blood and bone marrow responses to infec- peripheral blood) (Fig. 3.1). The neutrophils usually tion are non-specific and similar changes occur in show toxic granulation and may show Döhle bodies many other conditions, including trauma and other and cytoplasmic vacuolation. When there is very tissue damage, administration of growth factors, severe bacterial infection and in neonates, alco- carcinoma, Hodgkin’s disease, non-Hodgkin’s holics and patients with reduced bone marrow lymphoma and auto-immune disorders such as reserve, neutrophilia does not occur but there is a systemic lupus erythematosus. Only a minority of left shift with the above ‘toxic’ changes in neu- patients with an infection show peripheral blood or trophils. Certain bacterial infections, specifically bone marrow changes suggestive of a particular typhoid, paratyphoid and tularaemia, are character- micro-organism. ized by neutropenia rather than neutrophilia. In Fig. 3.1 PB, bacterial infection, left shift and toxic granulation. MGG ×940. 90 INFECTION AND REACTIVE CHANGES 91 Fig. 3.2 PB, bacterial infection, monocytosis and neutrophilia.