Cyanobacteria and Microalgae: a Positive Prospect for Biofuels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide
antioxidants Review Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide Ivan Kushkevych 1,* , Veronika Bosáková 1,2 , Monika Vítˇezová 1 and Simon K.-M. R. Rittmann 3,* 1 Department of Experimental Biology, Faculty of Science, Masaryk University, 62500 Brno, Czech Republic; [email protected] (V.B.); [email protected] (M.V.) 2 Department of Biology, Faculty of Medicine, Masaryk University, 62500 Brno, Czech Republic 3 Archaea Physiology & Biotechnology Group, Department of Functional and Evolutionary Ecology, Universität Wien, 1090 Vienna, Austria * Correspondence: [email protected] (I.K.); [email protected] (S.K.-M.R.R.); Tel.: +420-549-495-315 (I.K.); +431-427-776-513 (S.K.-M.R.R.) Abstract: Hydrogen sulfide is a toxic compound that can affect various groups of water microorgan- isms. Photolithotrophic sulfur bacteria including Chromatiaceae and Chlorobiaceae are able to convert inorganic substrate (hydrogen sulfide and carbon dioxide) into organic matter deriving energy from photosynthesis. This process takes place in the absence of molecular oxygen and is referred to as anoxygenic photosynthesis, in which exogenous electron donors are needed. These donors may be reduced sulfur compounds such as hydrogen sulfide. This paper deals with the description of this metabolic process, representatives of the above-mentioned families, and discusses the possibility using anoxygenic phototrophic microorganisms for the detoxification of toxic hydrogen sulfide. Moreover, their general characteristics, morphology, metabolism, and taxonomy are described as Citation: Kushkevych, I.; Bosáková, well as the conditions for isolation and cultivation of these microorganisms will be presented. V.; Vítˇezová,M.; Rittmann, S.K.-M.R. -

Limits of Life on Earth Some Archaea and Bacteria
Limits of life on Earth Thermophiles Temperatures up to ~55C are common, but T > 55C is Some archaea and bacteria (extremophiles) can live in associated usually with geothermal features (hot springs, environments that we would consider inhospitable to volcanic activity etc) life (heat, cold, acidity, high pressure etc) Thermophiles are organisms that can successfully live Distinguish between growth and survival: many organisms can survive intervals of harsh conditions but could not at high temperatures live permanently in such conditions (e.g. seeds, spores) Best studied extremophiles: may be relevant to the Interest: origin of life. Very hot environments tolerable for life do not seem to exist elsewhere in the Solar System • analogs for extraterrestrial environments • `extreme’ conditions may have been more common on the early Earth - origin of life? • some unusual environments (e.g. subterranean) are very widespread Extraterrestrial Life: Spring 2008 Extraterrestrial Life: Spring 2008 Grand Prismatic Spring, Yellowstone National Park Hydrothermal vents: high pressure in the deep ocean allows liquid water Colors on the edge of the at T >> 100C spring are caused by different colonies of thermophilic Vents emit superheated water (300C or cyanobacteria and algae more) that is rich in minerals Hottest water is lifeless, but `cooler’ ~50 species of such thermophiles - mostly archae with some margins support array of thermophiles: cyanobacteria and anaerobic photosynthetic bacteria oxidize sulphur, manganese, grow on methane + carbon monoxide etc… Sulfolobus: optimum T ~ 80C, minimum 60C, maximum 90C, also prefer a moderately acidic pH. Live by oxidizing sulfur Known examples can grow (i.e. multiply) at temperatures which is abundant near hot springs. -

Coral Feeding on Microalgae Assessed with Molecular Trophic Markers
Molecular Ecology (2013) doi: 10.1111/mec.12486 Coral feeding on microalgae assessed with molecular trophic markers MIGUEL C. LEAL,*† CHRISTINE FERRIER-PAGES,‡ RICARDO CALADO,* MEGAN E. THOMPSON,† MARC E. FRISCHER† and JENS C. NEJSTGAARD† *Departamento de Biologia & CESAM, Universidade de Aveiro, Campus Universitario de Santiago, 3810-193 Aveiro, Portugal, †Skidaway Institute of Oceanography, 10 Ocean Science Circle, 31411 Savannah, GA, USA, ‡Centre Scientifique de Monaco, Avenue St-Martin, 98000 Monaco, Monaco Abstract Herbivory in corals, especially for symbiotic species, remains controversial. To investi- gate the capacity of scleractinian and soft corals to capture microalgae, we conducted controlled laboratory experiments offering five algal species: the cryptophyte Rhodo- monas marina, the haptophytes Isochrysis galbana and Phaeocystis globosa, and the diatoms Conticribra weissflogii and Thalassiosira pseudonana. Coral species included the symbiotic soft corals Heteroxenia fuscescens and Sinularia flexibilis, the asymbiotic scleractinian coral Tubastrea coccinea, and the symbiotic scleractinian corals Stylophora pistillata, Pavona cactus and Oculina arbuscula. Herbivory was assessed by end-point PCR amplification of algae-specific 18S rRNA gene fragments purified from coral tissue genomic DNA extracts. The ability to capture microalgae varied with coral and algal species and could not be explained by prey size or taxonomy. Herbivory was not detected in S. flexibilis and S. pistillata. P. globosa was the only algal prey that was never captured by any coral. Although predation defence mechanisms have been shown for Phaeocystis spp. against many potential predators, this study is the first to suggest this for corals. This study provides new insights into herbivory in symbiotic corals and suggests that corals may be selective herbivorous feeders. -

PROTISTS Shore and the Waves Are Large, Often the Largest of a Storm Event, and with a Long Period
(seas), and these waves can mobilize boulders. During this phase of the storm the rapid changes in current direction caused by these large, short-period waves generate high accelerative forces, and it is these forces that ultimately can move even large boulders. Traditionally, most rocky-intertidal ecological stud- ies have been conducted on rocky platforms where the substrate is composed of stable basement rock. Projec- tiles tend to be uncommon in these types of habitats, and damage from projectiles is usually light. Perhaps for this reason the role of projectiles in intertidal ecology has received little attention. Boulder-fi eld intertidal zones are as common as, if not more common than, rock plat- forms. In boulder fi elds, projectiles are abundant, and the evidence of damage due to projectiles is obvious. Here projectiles may be one of the most important defi ning physical forces in the habitat. SEE ALSO THE FOLLOWING ARTICLES Geology, Coastal / Habitat Alteration / Hydrodynamic Forces / Wave Exposure FURTHER READING Carstens. T. 1968. Wave forces on boundaries and submerged bodies. Sarsia FIGURE 6 The intertidal zone on the north side of Cape Blanco, 34: 37–60. Oregon. The large, smooth boulders are made of serpentine, while Dayton, P. K. 1971. Competition, disturbance, and community organi- the surrounding rock from which the intertidal platform is formed zation: the provision and subsequent utilization of space in a rocky is sandstone. The smooth boulders are from a source outside the intertidal community. Ecological Monographs 45: 137–159. intertidal zone and were carried into the intertidal zone by waves. Levin, S. A., and R. -

Microalgae Strain Catalogue
Microalgae strain catalogue A strain selection guide for microalgae users: cultivation and chemical characteristics for high added-value products CONTENTS 1. INTRODUCTION .......................................................................................... 4 2. Strain catalogue ......................................................................................... 5 Anabaena cylindrica ........................................................................................... 6 Arthrospira platensis .......................................................................................... 8 Botryococcus braunii ........................................................................................ 10 Chlorella luteoviridis ......................................................................................... 12 Chlorella sorokiniana ........................................................................................ 14 Chlorella vulgaris .............................................................................................. 16 Dunaliella salina ............................................................................................... 18 Dunaliella tertiolecta ......................................................................................... 20 Haematococcus pluvialis .................................................................................. 22 Microcystis aeruginosa ..................................................................................... 24 Nannochloropsis occulata ............................................................................... -

21 Pathogens of Harmful Microalgae
21 Pathogens of Harmful Microalgae RS. Salomon and I. Imai 2L1 Introduction Pathogens are any organisms that cause disease to other living organisms. Parasitism is an interspecific interaction where one species (the parasite) spends the whole or part of its life on or inside cells and tissues of another living organism (the host), from where it derives most of its food. Parasites that cause disease to their hosts are, by definition, pathogens. Although infection of metazoans by other metazoans and protists are the more fre quently studied, there are interactions where both host and parasite are sin gle-celled organisms. Here we describe such interactions involving microal gae as hosts. The aim of this chapter is to review the current status of research on pathogens of harmful microalgae and present future perspec tives within the field. Pathogens with the ability to impair and kill micro algae include viruses, bacteria, fungi and a number of protists (see reviews by Elbrachter and Schnepf 1998; Brussaard 2004; Park et al. 2004; Mayali and Azam 2004; Ibelings et al. 2004). Valuable information exists from non-harm ful microalgal hosts, and these studies will be referred to throughout the text. Nevertheless, emphasis is given to cases where hosts are recognizable harmful microalgae. 21.2 Viruses Viruses and virus-like particles (VLPs) have been found in more than 50 species of eukaryotic microalgae, and several of them have been isolated in laboratory cultures (Brussaard 2004; Nagasaki et al. 2005). These viruses are diverse both in size and genome type, and some of them infect harmful algal bloom (HAB)-causing species (Table 21.1). -

Algae and Cyanobacteria in Coastal and Estuarine Waters
CHAPTER 7 Algae and cyanobacteria in coastal and estuarine waters n coastal and estuarine waters, algae range from single-celled forms to the seaweeds. ICyanobacteria are organisms with some characteristics of bacteria and some of algae. They are similar in size to the unicellular algae and, unlike other bacteria, contain blue-green or green pigments and are able to perform photosynthesis; thus, they are also termed blue-green algae. Algal blooms in the sea have occurred throughout recorded history but have been increasing during recent decades (Anderson, 1989; Smayda, 1989a; Hallegraeff, 1993). In several areas (e.g., the Baltic and North seas, the Adriatic Sea, Japanese coastal waters and the Gulf of Mexico), algal blooms are a recurring phe- nomenon. The increased frequency of occurrence has accompanied nutrient enrich- ment of coastal waters on a global scale (Smayda, 1989b). Blooms of non-toxic phytoplankton species and mass occurrences of macro-algae can affect the amenity value of recreational waters due to reduced transparency, dis- coloured water and scum formation. Furthermore, bloom degradation can be accom- panied by unpleasant odours, resulting in aesthetic problems (see chapter 9). Several human diseases have been reported to be associated with many toxic species of dinoflagellates, diatoms, nanoflagellates and cyanobacteria that occur in the marine environment (CDC, 1997). The effects of these algae on humans are due to some of their constituents, principally algal toxins. Marine algal toxins become a problem primarily because they may concentrate in shellfish and fish that are subsequently eaten by humans (CDR, 1991; Lehane, 2000), causing syndromes known as para- lytic shellfish poisoning (PSP), diarrhetic shellfish poisoning (DSP), amnesic shellfish poisoning (ASP), neurotoxic shellfish poisoning (NSP) and ciguatera fish poisoning (CFP). -
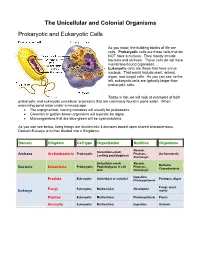
The Unicellular and Colonial Organisms Prokaryotic And
The Unicellular and Colonial Organisms Prokaryotic and Eukaryotic Cells As you know, the building blocks of life are cells. Prokaryotic cells are those cells that do NOT have a nucleus. They mostly include bacteria and archaea. These cells do not have membrane-bound organelles. Eukaryotic cells are those that have a true nucleus. That would include plant, animal, algae, and fungal cells. As you can see, to the left, eukaryotic cells are typically larger than prokaryotic cells. Today in lab, we will look at examples of both prokaryotic and eukaryotic unicellular organisms that are commonly found in pond water. When examining pond water under a microscope… The unpigmented, moving microbes will usually be protozoans. Greenish or golden-brown organisms will typically be algae. Microorganisms that are blue-green will be cyanobacteria. As you can see below, living things are divided into 3 domains based upon shared characteristics. Domain Eukarya is further divided into 4 Kingdoms. Domain Kingdom Cell type Organization Nutrition Organisms Absorb, Unicellular-small; Prokaryotic Photsyn., Archaeacteria Archaea Archaebacteria Lacking peptidoglycan Chemosyn. Unicellular-small; Absorb, Bacteria, Prokaryotic Peptidoglycan in cell Photsyn., Bacteria Eubacteria Cyanobacteria wall Chemosyn. Ingestion, Eukaryotic Unicellular or colonial Protozoa, Algae Protista Photosynthesis Fungi, yeast, Fungi Eukaryotic Multicellular Absorption Eukarya molds Plantae Eukaryotic Multicellular Photosynthesis Plants Animalia Eukaryotic Multicellular Ingestion Animals Prokaryotic Organisms – the archaea, non-photosynthetic bacteria, and cyanobacteria Archaea - Microorganisms that resemble bacteria, but are different from them in certain aspects. Archaea cell walls do not include the macromolecule peptidoglycan, which is always found in the cell walls of bacteria. Archaea usually live in extreme, often very hot or salty environments, such as hot mineral springs or deep-sea hydrothermal vents. -

About Cyanobacteria BACKGROUND Cyanobacteria Are Single-Celled Organisms That Live in Fresh, Brackish, and Marine Water
About Cyanobacteria BACKGROUND Cyanobacteria are single-celled organisms that live in fresh, brackish, and marine water. They use sunlight to make their own food. In warm, nutrient-rich environments, microscopic cyanobacteria can grow quickly, creating blooms that spread across the water’s surface and may become visible. Because of the color, texture, and location of these blooms, the common name for cyanobacteria is blue-green algae. However, cyanobacteria are related more closely to bacteria than to algae. Cyanobacteria are found worldwide, from Brazil to China, Australia to the United States. In warmer climates, these organisms can grow year-round. Scientists have called cyanobacteria the origin of plants, and have credited cyanobacteria with providing nitrogen fertilizer for rice and beans. But blooms of cyanobacteria are not always helpful. When these blooms become harmful to the environment, animals, and humans, scientists call them cyanobacterial harmful algal blooms (CyanoHABs). Freshwater CyanoHABs can use up the oxygen and block the sunlight that other organisms need to live. They also can produce powerful toxins that affect the brain and liver of animals and humans. Because of concerns about CyanoHABs, which can grow in drinking water and recreational water, the U.S. Environmental Protection Agency (EPA) has added cyanobacteria to its Drinking Water Contaminant Candidate List. This list identifies organisms and toxins that EPA considers to be priorities for investigation. ASSESSING THE IMPACT ON PUBLIC HEALTH Reports of poisonings associated with CyanoHABs date back to the late 1800s. Anecdotal evidence and data from laboratory animal research suggest that cyanobacterial toxins can cause a range of adverse human health effects, yet few studies have explored the links between CyanoHABs and human health. -

Rhythmicity of Coastal Marine Picoeukaryotes, Bacteria and Archaea Despite Irregular Environmental Perturbations
Rhythmicity of coastal marine picoeukaryotes, bacteria and archaea despite irregular environmental perturbations Stefan Lambert, Margot Tragin, Jean-Claude Lozano, Jean-François Ghiglione, Daniel Vaulot, François-Yves Bouget, Pierre Galand To cite this version: Stefan Lambert, Margot Tragin, Jean-Claude Lozano, Jean-François Ghiglione, Daniel Vaulot, et al.. Rhythmicity of coastal marine picoeukaryotes, bacteria and archaea despite irregular environmental perturbations. ISME Journal, Nature Publishing Group, 2019, 13 (2), pp.388-401. 10.1038/s41396- 018-0281-z. hal-02326251 HAL Id: hal-02326251 https://hal.archives-ouvertes.fr/hal-02326251 Submitted on 19 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Rhythmicity of coastal marine picoeukaryotes, bacteria and archaea despite irregular environmental perturbations Stefan Lambert, Margot Tragin, Jean-Claude Lozano, Jean-François Ghiglione, Daniel Vaulot, François-Yves Bouget, Pierre Galand To cite this version: Stefan Lambert, Margot Tragin, Jean-Claude Lozano, Jean-François Ghiglione, Daniel -

CYANOBACTERIA (BLUE-GREEN ALGAE) BLOOMS When in Doubt, It’S Best to Keep Out!
Cyanobacteria Blooms FAQs CYANOBACTERIA (BLUE-GREEN ALGAE) BLOOMS When in doubt, it’s best to keep out! What are cyanobacteria? Cyanobacteria, also called blue-green algae, are microscopic organisms found naturally in all types of water. These single-celled organisms live in fresh, brackish (combined salt and fresh water), and marine water. These organisms use sunlight to make their own food. In warm, nutrient-rich (high in phosphorus and nitrogen) environments, cyanobacteria can multiply quickly, creating blooms that spread across the water’s surface. The blooms might become visible. How are cyanobacteria blooms formed? Cyanobacteria blooms form when cyanobacteria, which are normally found in the water, start to multiply very quickly. Blooms can form in warm, slow-moving waters that are rich in nutrients from sources such as fertilizer runoff or septic tank overflows. Cyanobacteria blooms need nutrients to survive. The blooms can form at any time, but most often form in late summer or early fall. What does a cyanobacteria bloom look like? You might or might not be able to see cyanobacteria blooms. They sometimes stay below the water’s surface, they sometimes float to the surface. Some cyanobacteria blooms can look like foam, scum, or mats, particularly when the wind blows them toward a shoreline. The blooms can be blue, bright green, brown, or red. Blooms sometimes look like paint floating on the water’s surface. As cyanobacteria in a bloom die, the water may smell bad, similar to rotting plants. Why are some cyanbacteria blooms harmful? Cyanobacteria blooms that harm people, animals, or the environment are called cyanobacteria harmful algal blooms. -

The Cells That Changed the World (Adapted from the 2003 Windows to the Sea Expedition)
Thunder Bay Sinkholes 2008 The Cells That Changed the World (adapted from the 2003 Windows to the Sea Expedition) FOCUS SEATIN G ARRAN G E M ENT Cyanobacteria Classroom style or groups of three to four students GRADE LEVE L MAXI M U M NU M BER O F STUDENTS 9-12 (LIfe Science) 30 FOCUS QUESTION KEY WORDS What are cyanobacteria, and how have they Cold seeps been important to life on Earth? Cyanobacteria Photosynthesis LEARNIN G OBJECTIVES Chemosynthesis Students will compare and contrast cyanobacteria with eukaryotic algae. BAC kg ROUND IN F OR M ATION In June, 2001, the Ocean Explorer Thunder Bay Students will discuss differences in photosynthesis ECHO Expedition was searching for shipwrecks by cyanobacteria and green algae. in the deep waters of the Thunder Bay National Marine Sanctuary and Underwater Preserve in Students will discuss the role of cyanobacteria in Lake Huron. But the explorers discovered more the development of life on Earth. than shipwrecks: dozens of underwater sinkholes in the limestone bedrock, some of which were MATERIA L S several hundred meters across and 20 meters Copies of “Cyanobacteria Discovery deep. The following year, an expedition to sur- Worksheet,” one copy for each student or stu- vey the sinkholes found that some of them were dent group releasing fluids that produced a visible cloudy (Optional) Cultures of live cyanobacteria and layer above the lake bottom, and the lake floor materials for microscopic examination near some of the sinkholes was covered by con- spicuous green, purple, white, and brown mats. AUDIOVISUA L MATERIA L S None Preliminary studies of the mats have found that where water is shallow (≤ 1.0 m) the mats are TEACHIN G TI M E composed of green algae.