Optimum Ph Value for Improving Postharvest Characteristics and Extending Vase Life of Rosa Hybrida Cv
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Marion Garden Rose Garden
Marion Garden Rose Garden The Rose Garden is a work in constant change to demonstrate how to grow and maintain a variety of roses that are suitable for home gardens in the Willamette Valley. Selection of plants has been based on suitability for landscape use, resistance to disease, repeat blooming, and availability to the home gardener. If we find that a rose bush has a lot of disease problems or does not bloom well it is removed. The visitor will find mostly hybrid teas but also floribundas, grandifloras, shrub roses and an English (Austin) rose. There is also one upright climbing rose named ‘Antique’. Two clematis vines (Viola and Asso) have been planted on either side of the climbing rose trellis. Clematis are a good compliment to large climbing roses and add interest and color to the garden. All the rose bushes have ID tags which include the name of the rose, type, any awards it has received, name of the hybridizer, and the year the rose was introduced. There are a couple of roses that do not have ID tags because they were donated and names were not known. They are good disease resistant plants with a good bloom habit so are used for cuttings for rose propagation workshops. There is also a Lonicera fragrantissima (winter honeysuckle, January jasmine, or Chinese honeysuckle) growing on the northwest corner of the rose garden. It has fragrant white flowers in January and February. It was there when this garden was started and is a nice shrub for the home garden. It is pruned in the spring after it finishes blooming. -

CLIMBING These Are Roses That Have a Strong Vertical Growth Habit
CLIMBING These are roses that have a strong vertical growth habit. They must however, be trained on an arbor or trellis. Roses are genetically ROSE programmed to bloom at the end of their canes. To encourage more flowering shoots, PRICE GUIDE 2019 rose canes should be trained horizontally. This is why rambling roses do so well when Milaeger’s offers hundreds of varieties of top grown along a fence, and why climbers look so quality roses that are proven performers in our climate. This guide describes all of the beautiful when carefully twisted around a varieties that we are planning on offering this pillar, but often look sparse when forced year. These “Number One” grade, two-year- straight up a trellis. Because roses are always old plants are all hand-potted in large pots, losing and gaining wood, permanent ties are using only the finest materials, to ensure your impractical. It is best to weave canes through planting success. Roses add beauty and trelliswork or along a fence as they grow. romance to almost any sunny landscape setting, Sturdy twist-ties can also be used. Working and they are now easier than ever to grow. around thorny roses requires caution; Your input into our selection of varieties is goatskin gloves help because rose thorns always welcome. Please use this guide not only cannot penetrate them. A climber in for basic information, but to guide you through Wisconsin usually reaches between 6 and 10 the rose section on our retail lot. “Own root” and rugosas are marked as such. “Own root” feet tall and most growth occurs the second roses are propagated as a cutting rather than year. -

Best Roses for Cut Flowers - 2021 This List Is for Identifying Which Garden Roses Give Big Florist Buds and Long Stems
“A world of flowers, plants and a whole lot more.” Updated 3/26/2021 Best Roses for Cut Flowers - 2021 This list is for identifying which garden roses give big florist buds and long stems. Name of Rose Type Color Name of Rose Type Color About Face GR Gold/Orange Mister Lincoln HT Medium Red Abraham Darby DA Apricot/Yellow Moonstone HT White blend All My Loving HT Pink blend Munstead Wood DA Crimson Anna's Promise GR Golden Tan Neil Diamond HT Raspberry/White Barbra Streisand HT Deep Lavender Neptune HT Lavender blend Bewitched HT Medium Pink New Zealand HT Light Pink Boscobel DA Coral Octoberfest GR Orange blend Brandy HT Deep Apricot Oh My! FL Bright Red Brides Dream HT Pale Pink Oliva Rose Austin DA Soft Pink Charlotte DA Soft Yellow Olympiad HT Bright Red Colorific FL Salmon blend Over The Moon HT Apricot Darcey Bussel DA Deep Crimson Parade Day GR Pink/White Dick Clark GR Cherry Pink Perfect Moment HT Yellow/Red Dream Come True GR Yellow/Ruby Red Pope John Paul II HT White Easy Spirit FL White Princess Alexandra of Kent DA Deep Pink Elina HT Light Yellow Queen Elizabeth GR Medium Pink Elizabeth Taylor HT Deep Pink Radiant Perfume GR Deep Yellow Falling In Love HT Pink blend Rock & Roll GR Red/White Fragrant Plum GR Lavender blend Scepter'd Isle DA Sof Pink Full Sail HT White Secret HT Pink blend Gemini HT Pink blend Sedona HT Coral blend Gertrude Jekyll DA Pink Smokin' Hot HT Orange/Red Good as Gold HT Yellow blend Stainless Steel HT Silvery Lavender Graham Thomas DA Golden Yellow St. -

Overview of the Dynamic Role of Specialty Cut Flowers in the International Cut Flower Market
horticulturae Review Overview of the Dynamic Role of Specialty Cut Flowers in the International Cut Flower Market Anastasios Darras Laboratory of Floriculture and Landscape Architecture, Department of Agriculture, University of Peloponnese, 24100 Kalamata, Greece; [email protected]; Tel.: +30-27210-45199 Abstract: The global cut flower industry has faced serious challenges over the years, but still remains an important sector of agriculture. Floriculture businesses seek new, innovative trends and niches to help increase product sales. Specialty cut flower (SCF) production has increased in the past 20 years in the US, Australia, Africa, and Europe. SCF production and sales could increase further if these new products were supported by dynamic marketing campaigns that focus on their strengths compared to the traditional cut flowers (TCF) such as roses, carnations, gerberas, and chrysanthemums. The major strength of SCF is the eco-friendly profile, which is associated to low CO2 footprints and environmental outputs. This contrasts TCF cultivation, which is associated to high energy inputs, especially at the traditional production centres (e.g., The Netherlands). It is suggested that environmental legislations, production costs, and customer demand for eco-friendly products will positively affect future SCF cultivation and sale. Keywords: roses; gerberas; chrysanthemums; sustainability; floriculture; environmental impact; CO2 footprint Citation: Darras, A. Overview of the Dynamic Role of Specialty Cut 1. Introduction Flowers in the International Cut Global cut flower production and consumption has overcome serious challenges in the Flower Market. Horticulturae 2021, 7, past 20 years, especially those related to global economic recessions. The EU holds the first 51. https://doi.org/10.3390/ place in cut flower and ornamental potted plants sales with 31.0% of the global value, with horticulturae7030051 China and the USA in second and third place, holding 18.6% and 12.5%, respectively [1]. -

Rainbow Roses and Confetti Poinsettias
Gilding the Lilies: Rainbow Roses and Confetti Poinsettias António A. Monteiro, Roberto Lopez and Jules Janick RAINBOW ROSES Figure 1. Rainbow Roses. Others equally passionate say that artificial colo- ring creates new opportunities for decoration The colorful roses known as Rainbow Roses using natural flowers. However, proponents and shown on the cover of this issue of Chronica adversaries of this process both agree on the Horticulturae and in Fig. 1 were displayed in importance and enjoyment of cut flowers and several booths of Hortifair, a large flower show potted plants. Clearly, creating diversity must be held in Amsterdam, The Netherlands on considered a strength of horticultural science. October 9-12, 2007. Visitors were awestruck by the spectacular color combination and many could not explain how they were achieved. ABOUT THE AUTHORS Staining roses with dyes is a common practice to obtain flower colors that are not available in nature, as in the case of blue roses, the most common and first color to be used. However Figure 2. Confetti Poinsettia. Rainbow Roses are most unusual because the petals of the same flower display various colors. Combinations include “Ocean” roses with blue and yellow petals, and “Tropical” roses with yellow, orange and red petals. The technique for producing Rainbow Roses was developed by Peter van de Werken from River Roses®, a flower company located in António A. Roberto Lopez Holland. It is an elegant application of basic Monteiro knowledge of plant anatomy. However, the practical use of the method requires specific know-how in order to get an even distribution of the dye over the petal surface, avoiding color create a confetti splashing effect. -

Tips for Displaying Cut Flowers
TIPS FOR DISPLAYING CUT FLOWERS.... at home or at the fair 1. Remove the foliage from the portion of the stems that will be in water. Foliage covered with water will rot, discolor the water, and the bacteria growing in the water will block the stems causing the flowers to wilt sooner. 2. Labeling While bouquets at home don’t need labels, exhibits for the fair do. List the cultivar name as well as the type of flower (or vegetable) on the entry tag. Keeping records of what you plant and where will help you to know what each cultivar is when you prepare your exhibits. Exhibits without the cultivar or variety name are usually lowered one ribbon placing. Proper labeling insures that the judge can base the placing on the proper criteria. Good records also help you as you determine whether or not a specific cultivar is worth growing again. Finally, fair visitors and other exhibitors may be interested in growing the cultivars of plants that you grew and can get the information from the label. 3. Conditioning Flowers Conditioning flowers helps them last longer by reducing stem blockage and promoting quick water uptake. The vase life of many flowers, especially roses, can be increased by re-cutting the stems while holding them under water. This method prevents air from getting into the stem and blocking water uptake. Since you’ll need to re-cut the stems, always cut them as long as possible when harvesting your flowers. Cut the ends diagonally so they won’t rest flat on the bottom of the container. -

Kordes Roses Catalog
Your supplier of Kordes roses: Palatine Roses - Largest selection of disease free KORDES roses in north america 2108 Creek Road, RR# 3 Email [email protected] Niagara-On-The-Lake, ON L0S 1J0 Canada Website: www.palatineroses.com Tel. (905) 468-8627 Fax (905) 468-8628 Ashdown Roses Ltd. Pickering Nurseries, Inc. 2220 S. Blackstock Rd. 3043 County Rd. 2, R.R. # 1 Landrum, SC 29356 USA Port Hope, ON L1A 3V5 Canada Tel. (864) 468-4900 Tel. (866) 269-9282 Fax. (864) 468-4889 Fax. (905) 839-4807 Email: [email protected] Email. [email protected] Web: www.ashdownroses.com Web: www.pickeringnurseries.com Edmunds‘ Roses / Jung Seed Company Roses Unlimited 535 South High Street 363 N. Deerwood Drive Randolph, WI 53956 Laurens, SC 29360 USA Tel. (920) 326-3121 Tel. (864) 682-7673 Tel. (800) 347-7609 Fax. (864) 682-2455 Fax. (800) 374-6120 Email: [email protected] www.edmundsroses.com Web: www.rosesunlimitedownroot.com Heirloom Roses, Inc. Jackson & Perkins Roses / 24062 NE Riverside Drive Wayside Gardens / Park Seed St. Paul, Oregon 97137 USA 1 Garden Lane Tel. (503) 538-1576 Hodges, SC 29695 USA Fax. (503) 538-5902 Tel. (800) 845-1124 Email: [email protected] Fax. (864) 941-4502 Web: www.heirloomroses.com Email: [email protected] Northland Rosarium Web: www.waysidegardens.com 9405 S. Williams Lane Weeks Wholesale Rose Grower, Inc. Spokane, WA 99224 USA 30135 McCombs Road Tel. (509) 448-4968 Fax. (509) 443-2202 Wasco, CA 93280 Web: http://www.weeksroses.com Email: [email protected] Web: www.northlandrosarium.com W. Kordes’ Söhne Rosenschulen GmbH & Co KG Newfl ora LLC 972 Old Stage Road, Rosenstraße 54, 25365 Kl.Offens. -

SAY IT with a ROSE: a ROSE for EVERY OCCASION Key: Cut Flower - Mild Fragrance - Strong Fragrance
SAY IT WITH A ROSE: A ROSE FOR EVERY OCCASION Key: Cut flower - Mild fragrance - Strong fragrance BEST FRIEND Hybrid Tea A stylish, classic rose of great beauty and richness. Vibrant $25.95 blooms of deep, hot plum pink have a strong sweet perfume. The bush also features attractive, large, light green leaves. 1.5m tall RSPCA named to honour unconditional special friendship that comes from loving a pet. FATHER’S LOVE Hybrid Tea This rich, velvety, dark red rose is fully petalled, carrying an incredible, long lasting fragrance of spice. Deep green, $25.95 glossy foliage with good disease resistance. Named in support of men across Australia through 'Centre for Men’s Health'. MOTHER’S LOVE Hybrid Tea The soft white to creamy pink blooms gently deepen in colour toward the centre are mainly produced in clusters of $25.95 three. Strong, sweet fragrance. Abundant deep green foliage on a medium to tall, healthy bush reaching 1.5m. Supporting the Nursing Mother's Association of Australia MOTHER & DAUGHTER Hybrid Tea Large, soft-sulphur yellow double blooms on long, elegant $26.95 stems, make excellent cut flowers. Disease resistant with a wonderful scent. A ROSE FOR EVERY OCCASION PEACE Hybrid Tea The large exhibition blooms of yellow, flushed pink have a pleasant fragrance. Probably the most popular of all roses $17.95 and was the first rose inducted into the Rose Hall of Fame in 1976. SPIRIT OF PEACE Hybrid Tea Lovely well formed flowers in a very unusual colour of dark cream buff. Plant habit is medium tall, with very light green $17.95 stems and foliage. -

Ludwig's Roses Cape Town
R 5.00 From the editor THE TASCHNER FAMILY 46 YEARS OF ROSE PASSION A lot has changed since I first settled in Haakdoorn- laagte in 1971. One thing however has not. The rose remains the Queen of all flowers. In those days gardeners were intrigued by the first proper orange Hybrid Tea roses. They were beautiful, but riddled with mildew. Pamela & Ludwig The quest for breeding healthier roses has paid off. Today, due to the fungus disease resistance in modern roses, we enjoy many more blooms each season. Stamina™ roses are a step up. We are excited to introduce this generation of vigorous and robust cut rose bearing bushes. Vanessa, Little Ludwig, Otto & Halmar Their broad leaf canopy shades, nourishes and enables a wide and deeply settled root system. This creates a perfect circle of stamina and repeat flowering. ‘Fordyce’ and ‘Turning Point’ are this season’s “must have” Stamina™ novelties. Christian, Xavier, Alex & Heike Gardening is so much fun! Planting roses enhances the experience! Wishing you the rewarding joy each and every bloom brings! Yours in Roses, Ludwig Taschner & family Anja, Sofia, Talhat & Gabriel Pictures and information in this catalogue are intended as a guide only. Copyright © 2017/18 in text: Ludwig Taschner. Copyright © 2017/18 in all photographs: Ludwig Taschner. CONTENT Rose Use Symbology ..........................1 Trellis Prices ................................2 Suitable to be trained onto a trellis A very specific characteristicL that Ludwig finds charming. List of Novelty Roses .................2 t Events ...............................2 -
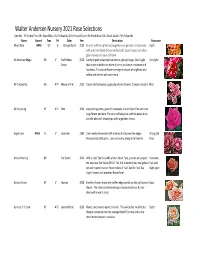
Walter Andersen Nursery 2021 Rose Selections
Walter Andersen Nursery 2021 Rose Selections Type Key - HT=Hybrid Tea, GR= Grandiflora, FL=Floribunda, GCV=Ground Cover, SH=Shrub Rose, DA= David Austin, PLY=Polyantha Name Awards Type Ht Color Year Description Fragrance About Face AARS GR 6' Orange blend 2005 Bi-color with the lighter lasting golden-orange color on the inside Slight with a distinct darker bronzy-red outside. Superb vigor, lush clean green leaves and loads of bloom. All American Magic GR 5' Red/Yellow 2010 Candy striped red and yellow blooms, glossy foliage. Less fragile Very light Stripe than other striped roses when it comes to disease resistance and hardiness. The double flowers emerge in stripes of bright red and yellow and fade to pink and cream. All Dressed Up GR 4'-5' Medium Pink 2019 Classic old-fashioned, cupped/quartered flowers. Disease resistant. Mild All My Loving HT 4'-5' Pink 2016 Long cutting stems, great for bouquets. A true Hybrid Tea with one large flower per stem. The color will stay true until the petals drop and the plant will reward you with large clean leaves. Angel Face AARS FL 3' Lavender 1969 Clear medium lavender with blushes of ruby near the edges. Strong Old Produces lots of blooms. Low and bushy, a long time favorite. Rose Anna's Promise GR Tan blend 2014 With a ‘dad’ like the AARS winner About Face, you can only expect Moderate the new rose ‘kid’ to be GREAT. The ‘kid’ inherited the novel golden fruit with tan with copper reverse flower colors of ‘dad’ but the ‘son’ has slight spice larger flowers and improved flower form. -

World Federation of Rose Societies 2014 Directory
WFRS WFRS • WFRS • WFRS WFRS • WFRS WORLD FEDERATION WFRS • WFRS OF ROSE SOCIETIES WFRS • WFRS WFRS • WFRS WFRS • WFRS 2014 DIRECTORY WFRS • WFRS WFRS • WFRS WFRS WFRS Executive Director • Mr. Malcolm Watson WFRS WFRS 29 Columbia Crescent • Modbury North WFRS WFRS SA 5092 • Australia WFRS WFRS Tel: (Country Code: 61) 8264 0084 • Email: [email protected] WFRS • WFRS WFRS • WFRS WFRS • WFRS WFRS • WFRS Table of Contents World Federation of Rose Societies 3 Breeders' Club 43 Argentina 46 Australia 50 Austria 67 Belgium 75 Bermuda 87 Canada 94 Chile 113 China 119 Czech Republic 121 Denmark 128 Finland 145 France 150 Germany 165 Greece 179 Hungary 182 Iceland 183 India 187 Israel 199 Italy 202 Japan 215 Luxembourg 234 Monaco 238 Netherlands 240 New Zealand 246 Northern Ireland 262 Norway 268 Pakistan 273 Romania 282 Russia 292 Serbia 295 Slovakia 296 Slovenia 305 South Africa 309 Spain 317 Sweden 324 Switzerland 337 United Kingdom 351 United States of America 369 Uruguay 405 WORLD FEDERATION OF ROSE SOCIETIES WORLD FEDERATION OF ROSE SOCIETIES INTRODUCTION One of the most important functions of the World Federation of Rose Societies, as stated in our Constitution, is "To encourage and facilitate the interchange of information about and knowledge of the rose between national rose societies". The World Federation of Rose Societies Rose Directory attempts to do that. Our aim is to gather together the most important rose information from each of the thirty-nine member countries that make up the WFRS. This is information that is commonly known by members of each national rose society about roses in their own country, but it is information that is hard to come by for other rose lovers. -

2021 Rose List
2021 Rose List Cultivar/Variety Name Color Description Antique American Beauty ! Deep Pink 1875. Hybrid Perpetual. Cup-shaped flowers with a brilliant crimson color. Strong tea-scented blooms. Blooms heaviest in spring and fall. Thornless. Petal count 50. Blush Noisette ! Blush Pink 1817. Noisette. Soft pink double blooms are produced in clusters of 6 to 12. Light clove fragrance. Repeat blooming. Petal count of 25-40. CL Cecile Brunner ! Pink 1894. Polyantha Climber. Clusters of small pointed pastel pink buds open to reveal creamy pink blooms with a moderate tea fragrance. Blooms heaviest in spring on old wood; some reblooming in the fall. Petal count 25-40. CL Celine Forestier ! Yellow Blend 1858. Noisette Climber. Fully double blooms of soft pink, cream and yellow with a strong spicy scent. Petal count 25-40. CL Fortuniana ! White 1850. Fortuniana. Violet scented, two inch, double blooms, with a knotted center are borne on nearly thornless canes. Blooms in spring. Petal count 25-40. CL Mermaid ! Yellow 1918. Climber. Fragrant, large, single flowers. Repeats best once established. Petal count 5-7. CL Mme. Alfred Carriere ! White 1879. Noisette. A strong, reliable climbing rose. Large, sweetly scented, cupped blooms; creamy white tinted with pink. Flowers freely and continually. Petal Count 30. CL Old Blush ! Pink UNK. China Climber. Heavy bloomer and very hardy. Repeat bloomer. Petal count 9-15. CL Peggy Martin ! Pink UNK. Climber. Famous rose that survived Hurricane Katrina. Blooms throughout the growing season on nearly thornless canes. Petal count 15-25. CL Sombreuil ! White 1850. Tea. Creamy-white blooms are very large, flat, and quartered, with a most delicious tea rose fragrance.