Concentrating Solar Power
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report 2018 2
P HOTOVOLTAIC P OWER S YSTEMS P ROGRAMME ANNUAL REPORT 2018 2 COVER PHOTO: GREEN ENERGY PARK - A First Model in Africa The Green Energy Park photovoltaic plant, inaugurated by His Majesty King Mohammed VI in January 2017, is a unique model in Africa. The plant, with a total capacity of 250 kW, installed and connected to the grid is composed by different sub-systems with capacities varying between 5 kW and 30 kW. Its outdoor test platforms are designed in collaboration with the Fraunhofer CSP institute, and combine a multitude of test-set-ups, in order to investigate and characterize PV modules in harsh weather conditions and to obtain valuable data about their performances onsite. Located in the Green City of Ben Guerir, it covers the electrical needs of the Green Energy Park platform laboratories. It covers an area of approximately 1,5 ha. The plant, considered as a living laboratory by itself, contributes to R&D projects for the determination and identification of the most suited technologies for local conditions. It also gives the opportunity for national and worldwide PV module producers to test and characterize their photovoltaic technologies in real conditions. The plant is owned and operated by the Green Energy Park platform. KEY POINTS • Installed in self-consumption configuration to cover the R&D platform needs in terms of electricity; • Composed of many types of technologies (crystalline, thin film, CPV, fixed static structures, trackers); • Total area 1,5 ha; • 25 solar string inverters with different capacities are used and distributed for each sub-system; • Test of photovoltaic technologies at module level to identify the degradation mechanisms occurring on the different components of the PV modules in real conditions; • Test of photovoltaic technologies at string level to characterize their behavior in terms of power ratio and degradation mechanisms on the different component of PV plants in real local conditions. -

Hybrid Wind-Solar Reliable Solution for Turkey to Meet Electric Demand
BALKAN JOURNAL OF ELECTRICAL & COMPUTER ENGINEERING DOI: 10.17694/bajece.73922 62 Hybrid wind-solar reliable solution for Turkey to meet electric demand K. Dawood Abstract— Energy is the most important part of life, it is essential for social and economic development. Turkey is importing more than half of the energy from other countries to produce electricity by thermal plants. Air pollution is also becoming quite a big problem for Turkey due to the thermal production of the energy. One of the most effective solution for air pollution is renewable energy because nowadays renewable energy is environment Fig.1. Generation of electric power since 2011 [2] friendly. Turkey has many geographical location advantages one of them is renewable energy resources. Turkey has limited fossil fuel Figure 1 shows the generation of electricity in Turkey from resources and due to this reason Turkey must shift more electricity 2011 to 2015. Last year, more than 68 percent of total production to the renewable energy resources. Renewable energy has less environment impacts as compare to the fossil fuels but still electricity was generated by thermal power plants. renewable energy sources are not reliable and competitive as Approximately 5 percent of the electricity was generated from compare to the fossil fuels. The present study deals with the renewable assets of Turkey, 26 percent of electricity generated advantages of Hybrid renewable energy systems (Solar and Wind through hydropower plants. energy) in Turkey. Turkey is one of the richest country in the world in terms of Map of Turkey with high resources of solar-wind hybrid energy renewable resources. -

Interplay Between the Potential of Photovoltaic Systems And
1 Interplay between the potential of photovoltaic systems and 2 agricultural land use 3 Luís Dias*, João Pedro Gouveia, Paulo Lourenço, Júlia Seixas 4 5 CENSE – Center for Environmental and Sustainability Research, NOVA School of Science 6 and Technology, NOVA University Lisbon 7 8 *Corresponding author. Address: CENSE – Center for Environmental and Sustainability 9 Research, NOVA School of Science and Technology, NOVA University Lisbon 2829-516 10 Caparica, Portugal 11 Tel.:+351 21 294 83 74 12 E-mail address: [email protected] (Dias, L.) 13 14 Highlights 15 • Technical potential for utility-scale solar PV projects in rural areas is assessed. 16 • Agriculture and nature conservation land use shorten solar PV farms potential. 17 • Limited PV potential ca still cover substantial shares of local annual electricity consumption. 18 • 1 MW CPV projects show the highest land-use efficiency and productivity. 19 • PV contributes significantly to region energy independence. 20 21 Abstract 22 The recent decrease in solar photovoltaic (PV) investment cost has transformed the 23 attractiveness of the technology. Southern Europe has one of the highest levels of solar radiation 24 in the world, and policy makers are very keen to take full advantage of this resource for 25 electricity and heat production. However, physiographic characteristics and specific land uses 26 (e.g. agro-forestry and nature conservation) present important spatial constraints. This paper 27 proposes a methodology for the evaluation of utility-scale solar PV projects’ (>1 MW) technical 28 potential. The municipality of Évora (Portugal) was used as a case study, considering 29 topographical features and spatial planning regulations. -

Seragie Daniel 213016737.Pdf
An application of pinch analysis in the design of a stand-alone thermochemical cycle, hydrogen hub sourced from renewables in rural South Africa By Daniel Cecil Seragie Thesis submitted in fulfilment of the requirements for the degree Master of Engineering: Chemical Engineering In the Faculty of Engineering At the Cape Peninsula University of Technology Supervisor: Mr. Joe John Co-Supervisor: Prof Daniel Ikhu-Omoregbe Bellville Campus May 2020 CPUT copyright information The dissertation/thesis may not be published either in part (in scholarly, scientific or technical journals), or as a whole (as a monograph), unless permission has been obtained from the University DECLARATION I, Daniel Cecil Seragie, declare that the contents of this dissertation/thesis represent my unaided work and that the dissertation/thesis has not previously been submitted for academic examination towards any qualification. Furthermore, it represents my own opinions and not necessarily those of the Cape Peninsula University of Technology. Signed Date i | P a g e ABSTRACT Economic and social development are closely related to the accessibility of electricity. Meanwhile, non- grid connected rural areas shoulder the burden of health and environmental risks since extending the grid is considered uneconomical. Renewable hydrogen, hybrid energy systems are viewed as a promising solution in remote areas where grid extension is costly and fuel costs increase parallel to remoteness. Hence, this study applies heat and power pinch analysis in the conceptual design of an isolated, decentralized thermochemical cycle hydrogen & biogas energy hybrid, to satiate the electricity needs of a non-grid rural area in South Africa. This study highlights the value of using heat pinch and PoPA tools as a mid-term supplement to combat increasing energy costs, reduce negative environmental impacts, improve profits, and more importantly as a contribution to ensuring temperatures are kept well below 2℃ above pre-industrial levels. -

Advances in Concentrating Solar Thermal Research and Technology Related Titles
Advances in Concentrating Solar Thermal Research and Technology Related titles Performance and Durability Assessment: Optical Materials for Solar Thermal Systems (ISBN 978-0-08-044401-7) Solar Energy Engineering 2e (ISBN 978-0-12-397270-5) Concentrating Solar Power Technology (ISBN 978-1-84569-769-3) Woodhead Publishing Series in Energy Advances in Concentrating Solar Thermal Research and Technology Edited by Manuel J. Blanco Lourdes Ramirez Santigosa AMSTERDAM • BOSTON • HEIDELBERG LONDON • NEW YORK • OXFORD • PARIS • SAN DIEGO SAN FRANCISCO • SINGAPORE • SYDNEY • TOKYO Woodhead Publishing is an imprint of Elsevier Woodhead Publishing is an imprint of Elsevier The Officers’ Mess Business Centre, Royston Road, Duxford, CB22 4QH, United Kingdom 50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States The Boulevard, Langford Lane, Kidlington, OX5 1GB, United Kingdom Copyright © 2017 Elsevier Ltd. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publisher’s permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions. This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein). Notices Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary. -

Page 1 of 20 RSC Advances
RSC Advances This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. This Accepted Manuscript will be replaced by the edited, formatted and paginated article as soon as this is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/advances Page 1 of 20 RSC Advances Modelling and analysis on the effect of different parameters on a parabolic-trough concentrating solar system M.K. Islam ∗,a , M. Hasanuzzaman a, N.A. Rahim a,b aUM Power Energy Dedicated Advanced Centre (UMPEDAC), Level 4, Wisma R&D, University of Malaya, 59990 Kuala Lumpur, Malaysia b Renewable Energy Research Group, King Abdulaziz University, Jeddah 21589, Saudi Arabia Abstract Concentrating solar power technologies are potential energy-harvesting systems. This paper simulates and analyzes the design of a parabolic-trough concentrating solar system. Optimum measurements are sought for the receiver, and collector performance is investigated using three heat transfer fluids, namely, ammonia, nitrogen, and carbon dioxide. -

Efficiency Gain of the Solar Trough Receiver Using Different Optically Active Layers
EFFICIENCY GAIN OF THE SOLAR TROUGH RECEIVER USING DIFFERENT OPTICALLY ACTIVE LAYERS Khaled Shehata Baiuomy Mohamad Supervised by: Professor Philippe Ferrer University of Witwatersrand Johannesburg, 24th October 2019 A dissertation submitted for the fulfillment of the requirements of the degree of Doctor of Philosophy to the Faculty of Science, University of Witwatersrand i Declaration I declare that this thesis is my own, unaided work. It is being submitted for the Degree of Doctor of Philosophy at the University of the Witwatersrand, Johannesburg. It has not been submitted before for any degree or examination at any other University. Student: Khaled Shehata Baiuomy Mohamad Supervisor: Professor Philippe Ferrer ii Dedication To my family. iii Presentations arising from this study Paper Presentation, “Thermal performance analysis of novel alternative designs for parabolic trough solar collector,” 64th Annual Conference of the South African Institute of Physics (July 2019). (Granted an award for the best Ph.D. oral presentation in Applied Physics division) Paper Presentation, “Experimental and numerical study of a cavity and hot mirror receiver of the parabolic trough collector,” 63rd Annual Conference of the South African Institute of Physics (June 2018). Invited presentation, “Parabolic trough Efficiency gain through the use of a cavity absorber with a hot mirror,” Material and Energy research group, workshop, (November 2017). Invited Presentation, “Experimental and Numerical Heat Transfer Analysis of Cavity absorber and The Application of Different Optically Active Layer for Parabolic Solar Trough Concentrator,” Physics school, Wits University, (October 2017). Paper Presentation, “Experimental and Numerical Heat Transfer Analysis of Cavity absorber and The Application of Different Optically Active Layer for Parabolic Solar Trough Concentrator,” 62nd Annual Conference of the South African Institute of Physics (July 2017). -

3M Solar Technologies
3M Film Technologies Durable Films for Solar Light Management Tim Hebrink Staff Scientist 3M Company March 28, 2012 Photo courtesy of Ray Colby with Sundial Solar “Creating a brighter, more durable, more secure, and cleaner future” Hebrink Residence – Scandia Regional Lab 3M Film Technologies c-Si 3M Strategy for Solar Fabrication Reduced costs Thin Film Installation Cost Conversion kWh CPV Efficiency Light Management Increased energy Encapsulation output CSP Thermal management 3M Film Technologies 3M Solar Light Management Technologies Platforms Products Market Segments SMF 1100 Concentrated CSP Solar Power Electricity Generation Light Multilayer Industrial / Building Concentration Mirror Films Solar Thermal Heating Structural Low X Concentrated Panels CPV PhotoVoltaic Electricity Generation PhotoVoltaic and Surface Flat Panel Solar Thermal Structured Films/coatings Light Capture Weatherable Front Anti-Soil Thin Film and Back Surface Coatings Films Films Tapes Adhesives 3M Films/Tapes/Adhesives for c-Si Photovoltaic Junction Box Bonding Die cut of 3M™ Solar Acrylic Foam Tape or Bead of 3M™ PV 1000 Adhesive/Sealant Encapsulating 3M™ Scotchshield™ Film 17T (backside barrier film) Cosmetic Applications 3M™ Specialty Tapes Cell Positioning 3M™ Specialty Tapes Frame Bonding 3M™ Solar Acrylic Foam Tape Identification Solution 3M™ Performance Label Materials © 3M 2009. All Rights Reserved. 3M Film Technologies Multi-layer Optical Mirror Films ¼ Wave Constructive Interference Reflected waves – 100-1000 layers – 15-200 nm thick Incident wave – -
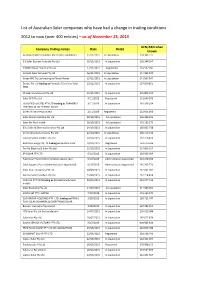
List of Australian Solar Companies Who Have Had a Change in Trading Conditions
List of Australian Solar companies who have had a change in trading conditions 2012 to now (over 400 entries) – as of November 25, 2015 ACN/ABN when Company trading names Date Detail known Australian Micro Inverters Pty Ltd (In Liquidation) 25/11/2015 In Liquidation 159 386 777 S.X Solar Express Australia Pty Ltd 19/11/2015 In Liquidation 126 348 507 CAIRNS VALUE SOLAR PTY LTD 17/11/2015 Registered 154 587 185 Armada Solar Services Pty Ltd 16/11/2015 In Liquidation 144 965 042 Green RRC Pty Ltd trading as Planet Power 13/11/2015 In Liquidation 141 584 947 Dorost Pty Ltd trading as Formerly T/as Clear Solar 12/11/2015 In Liquidation 137 609 851 NSW JP Solar Installations Pty Ltd 12/11/2015 In Liquidation 155 806 125 Solar DFO Pty Ltd 4/11/2015 Registered 151 646 203 QUALIFIED QUOTES PTY LTD trading as FORMERLY 3/11/2015 In Liquidation 165 356 294 TRADING AS NO DEPOSIT SOLAR Green Alliance Pty Limited 2/11/2015 Registered 122 821 363 Solar Winds Australia Pty Ltd 30/10/2015 In Liquidation 126 384 325 Solar Set Pty Limited 28/10/2015 In Liquidation 145 163 579 Elite Solar & Electrical Services Pty Ltd 24/10/2015 In Liquidation 166 865 738 Green Initiatives Services Pty Ltd 22/10/2015 In Liquidation 166 114 512 Sunrise Solar Installers Pty Ltd 20/10/2015 In Liquidation 132 218 850 Red Kite Energy Pty Ltd trading as Red Kite Solar 19/10/2015 Registered 163 525 344 Pro-Ro Electrics & Solar Pty Ltd 13/10/2015 In Liquidation 143 600 337 EP SOLAR PTY LTD 6/10/2015 In Liquidation 138 556 304 Free Solar Pty Ltd (Administrators Appointed) 6/10/2015 Administrators Appointed 131 248 336 Solar Support Pty Ltd (Administrators Appointed) 6/10/2015 Administrators Appointed 145 705 773 Solar City Enterprises Pty Ltd 30/09/2015 In Liquidation 142 641 281 Sunrise Solar Installers Pty Ltd 25/09/2015 In Liquidation 132 218 850 ACN 151 277 913 trading as PH Electrical & Solar 23/09/2015 In Liquidation 151 277 913 Installs Solar Economy Pty Ltd 14/09/2015 In Liquidation 144 963 959 SOLAR SET PTY LIMITED 7/09/2015 In Liquidation 145 163 579 SSW GROUP HOLDINGS PTY. -

REVIEW of RENEWABLE SOLAR ENERGY a Project Presented to The
REVIEW OF RENEWABLE SOLAR ENERGY A Project Presented to the faculty of the Department of Mechanical Engineering California State University, Sacramento Submitted in partial satisfaction of the requirements for the degree of MASTER OF SCIENCE in Mechanical Engineering by Usha Kiranmayee Bhamidipati SUMMER 2012 REVIEW OF RENEWABLE SOLAR ENERGY A Project by Usha Kiranmayee Bhamidipati Approved by: __________________________________, Committee Chair Dr. Dongmei Zhou ___________________________ Date ii Student: Usha Kiranmayee Bhamidipati I certify that this student has met the requirements for format contained in the University format manual, and that this project is suitable for shelving in the Library and credit is to be awarded for the thesis. __________________________, Graduate Coordinator ___________________ Dr. Akihiko Kumagai Date Department of Mechanical Engineering iii Abstract of REVIEW OF RENEWABLE SOLAR ENERGY by Usha Bhamidipati The major challenge that our planet is facing today is the anthropogenic driven climate changes and its link to our global society’s present and future energy needs. Renewable energy sources are now widely regarded as an important energy source. This technology contributes to the reduction of environmental impact, improved energy security and creating new energy industries. Traditional Fossil fuels such as oil, natural gas, coals are in great demand and are highly effective but at the same time they are damaging human health and environment. In terms of environment the traditional fossil fuels are facing a lot of pressure. The most serious challenge would perhaps be confronting the use of coal and natural gas while keeping in mind the greenhouse gas reduction target. It is now clear that in order to keep the levels of CO2 below 550 ppm, it cannot be achieved fundamentally on oil or coal based global economy. -

Turkey's Renewable Energy Sector from a Global Perspective
www.pwc.com/tr Turkey’s Renewable Energy Sector from a Global Perspective Welcome Over the past few years, policymakers in Turkey have realised the role that renewable energy can play in expanding power generation and diversifying the energy supply mix in an environmentally sustainable way. As Turkey’s reliance on imported More steps need to be taken in a Faruk Sabuncu natural gas for power generation has coordinated manner to improve Energy, Utilities and given rise to concerns over supply the investment environment for Mining Leader security and the country’s bulging renewable energy in Turkey. current account deficit, support of Firstly, regulatory uncertainties domestic energy sources such as coal and bureaucratic inefficiencies and renewables has gained a new in licensing and granting permits urgency. In this regard, Turkey’s shall be eliminated. Secondly, an new Renewable Energy Support investment plan shall be made for the Mechanism is an important step expansion of the power transmission forward. network in a way that will enable Murat Çolakoğlu the optimal mix of renewable energy Power Leader This publication is about Turkey’s power plants to be connected to the new Renewable Energy Support grid. More generally, policies that Mechanism and the state of play support the energy sector as a whole in its renewable energy sector. would also be beneficial for investors However, we also look at the policy in renewable energy. As such, further environment and commercial liberalization of the energy markets developments around the world, as would be key to attracting more we believe that the renewable energy investment to the energy sector. -

The Potential of Agrivoltaic Systems Harshavardhan Dinesh, Joshua Pearce
The Potential of Agrivoltaic Systems Harshavardhan Dinesh, Joshua Pearce To cite this version: Harshavardhan Dinesh, Joshua Pearce. The Potential of Agrivoltaic Systems. Renewable and Sus- tainable Energy Reviews, Elsevier, 2016, 54, pp.299-308. 10.1016/j.rser.2015.10.024. hal-02113575 HAL Id: hal-02113575 https://hal.archives-ouvertes.fr/hal-02113575 Submitted on 29 Apr 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Preprint of: Harshavardhan Dinesh, Joshua M. Pearce, The potential of agrivoltaic systems, Renewable and Sustainable Energy Reviews, 54, 299-308 (2016). DOI:10.1016/j.rser.2015.10.024 The Potential of Agrivoltaic Systems Harshavardhan Dinesh1 and Joshua M. Pearce1,2,* 1. Department of Electrical & Computer Engineering, Michigan Technological University 2. Department of Materials Science & Engineering, Michigan Technological University * Contact author: 601 M&M Building 1400 Townsend Drive Houghton, MI 49931-1295 906-487-1466 [email protected] Abstract: In order to meet global energy demands with clean renewable energy such as with solar photovoltaic (PV) systems, large surface areas are needed because of the relatively diffuse nature of solar energy. Much of this demand can be matched with aggressive building integrated PV and rooftop PV, but the remainder can be met with land-based PV farms.