Type of the Paper (Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcriptional Mechanisms of Resistance to Anti-PD-1 Therapy
Author Manuscript Published OnlineFirst on February 13, 2017; DOI: 10.1158/1078-0432.CCR-17-0270 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Transcriptional mechanisms of resistance to anti-PD-1 therapy Maria L. Ascierto1, Alvin Makohon-Moore2, 11, Evan J. Lipson1, Janis M. Taube3,4, Tracee L. McMiller5, Alan E. Berger6, Jinshui Fan6, Genevieve J. Kaunitz3, Tricia R. Cottrell4, Zachary A. Kohutek7, Alexander Favorov8,10, Vladimir Makarov7,11, Nadeem Riaz7,11, Timothy A. Chan7,11, Leslie Cope8, Ralph H. Hruban4,9, Drew M. Pardoll1, Barry S. Taylor11,12,13, David B. Solit13, Christine A Iacobuzio-Donahue2,11, and Suzanne L. Topalian5 From the 1Departments of Oncology, 3Dermatology, 4Pathology, 5Surgery, 6The Lowe Family Genomics Core, 8Oncology Bioinformatics Core, and the 9 Sol Goldman Pancreatic Cancer Research Center, Johns Hopkins University School of Medicine and Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD 21287; the 10Laboratory of System Biology and Computational Genetics, Vavilov Institute of General Genetics, Russian Academy of Sciences, 119991, Moscow, Russia; and 2Pathology, 7Radiation Oncology, 11Human Oncology and Pathogenesis Program, 12Department of Epidemiology and Biostatistics, and the 13Center for Molecular Oncology, Memorial Sloan Kettering Cancer Center, New York NY 10065. MLA, AM-M, EJL, and JMT contributed equally to this work Running title: Transcriptional mechanisms of resistance to anti-PD-1 Key Words: melanoma, cancer genetics, immunotherapy, anti-PD-1 Financial Support: This study was supported by the Melanoma Research Alliance (to SLT and CI-D), the Bloomberg~Kimmel Institute for Cancer Immunotherapy (to JMT, DMP, and SLT), the Barney Family Foundation (to SLT), Moving for Melanoma of Delaware (to SLT), the 1 Downloaded from clincancerres.aacrjournals.org on October 2, 2021. -

Hippo and Sonic Hedgehog Signalling Pathway Modulation of Human Urothelial Tissue Homeostasis
Hippo and Sonic Hedgehog signalling pathway modulation of human urothelial tissue homeostasis Thomas Crighton PhD University of York Department of Biology November 2020 Abstract The urinary tract is lined by a barrier-forming, mitotically-quiescent urothelium, which retains the ability to regenerate following injury. Regulation of tissue homeostasis by Hippo and Sonic Hedgehog signalling has previously been implicated in various mammalian epithelia, but limited evidence exists as to their role in adult human urothelial physiology. Focussing on the Hippo pathway, the aims of this thesis were to characterise expression of said pathways in urothelium, determine what role the pathways have in regulating urothelial phenotype, and investigate whether the pathways are implicated in muscle-invasive bladder cancer (MIBC). These aims were assessed using a cell culture paradigm of Normal Human Urothelial (NHU) cells that can be manipulated in vitro to represent different differentiated phenotypes, alongside MIBC cell lines and The Cancer Genome Atlas resource. Transcriptomic analysis of NHU cells identified a significant induction of VGLL1, a poorly understood regulator of Hippo signalling, in differentiated cells. Activation of upstream transcription factors PPARγ and GATA3 and/or blockade of active EGFR/RAS/RAF/MEK/ERK signalling were identified as mechanisms which induce VGLL1 expression in NHU cells. Ectopic overexpression of VGLL1 in undifferentiated NHU cells and MIBC cell line T24 resulted in significantly reduced proliferation. Conversely, knockdown of VGLL1 in differentiated NHU cells significantly reduced barrier tightness in an unwounded state, while inhibiting regeneration and increasing cell cycle activation in scratch-wounded cultures. A signalling pathway previously observed to be inhibited by VGLL1 function, YAP/TAZ, was unaffected by VGLL1 manipulation. -

Breakpoint Mapping and Haplotype Analysis of Translocation T(1;12)(Q43;Q21.1) in Two Apparently Independent Families with Vascular Phenotypes
Received: 7 August 2017 | Revised: 9 October 2017 | Accepted: 11 October 2017 DOI: 10.1002/mgg3.346 ORIGINAL ARTICLE Breakpoint mapping and haplotype analysis of translocation t(1;12)(q43;q21.1) in two apparently independent families with vascular phenotypes Tiia Maria Luukkonen1,2 | Mana M. Mehrjouy3 | Minna Poyh€ onen€ 4,5 | Anna-Kaisa Anttonen6 | Paivi€ Lahermo1 | Pekka Ellonen1 | Lars Paulin7 | Niels Tommerup3 | Aarno Palotie1,8 | Teppo Varilo2,5 1Institute for molecular medicine Finland FIMM, University of Helsinki, Helsinki, Abstract Finland Background: The risk of serious congenital anomaly for de novo balanced 2Department of Health, National Institute translocations is estimated to be at least 6%. We identified two apparently inde- for Health and Welfare, Helsinki, Finland pendent families with a balanced t(1;12)(q43;q21.1) as an outcome of a “System- 3Wilhelm Johannsen Centre for atic Survey of Balanced Chromosomal Rearrangements in Finns.” In the first Functional Genome Research, Department of Cellular and Molecular family, carriers (n = 6) manifest with learning problems in childhood, and later Medicine, University of Copenhagen, with unexplained neurological symptoms (chronic headache, balance problems, Copenhagen, Denmark tremor, fatigue) and cerebral infarctions in their 50s. In the second family, two 4Clinical Genetics, Helsinki University Hospital, University of Helsinki, carriers suffer from tetralogy of Fallot, one from transient ischemic attack and one Helsinki, Finland from migraine. The translocation cosegregates with these vascular phenotypes and 5Department of Medical Genetics, neurological symptoms. University of Helsinki, Helsinki, Finland Methods and Results: We narrowed down the breakpoint regions using mate 6Laboratory of Genetics, HUSLAB, Helsinki, Finland pair sequencing. We observed conserved haplotypes around the breakpoints, 7Institute of Biotechnology, University of pointing out that this translocation has arisen only once. -

Predict AID Targeting in Non-Ig Genes Multiple Transcription Factor
Downloaded from http://www.jimmunol.org/ by guest on September 26, 2021 is online at: average * The Journal of Immunology published online 20 March 2013 from submission to initial decision 4 weeks from acceptance to publication Multiple Transcription Factor Binding Sites Predict AID Targeting in Non-Ig Genes Jamie L. Duke, Man Liu, Gur Yaari, Ashraf M. Khalil, Mary M. Tomayko, Mark J. Shlomchik, David G. Schatz and Steven H. Kleinstein J Immunol http://www.jimmunol.org/content/early/2013/03/20/jimmun ol.1202547 Submit online. Every submission reviewed by practicing scientists ? is published twice each month by http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://www.jimmunol.org/content/suppl/2013/03/20/jimmunol.120254 7.DC1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 26, 2021. Published March 20, 2013, doi:10.4049/jimmunol.1202547 The Journal of Immunology Multiple Transcription Factor Binding Sites Predict AID Targeting in Non-Ig Genes Jamie L. Duke,* Man Liu,†,1 Gur Yaari,‡ Ashraf M. Khalil,x Mary M. Tomayko,{ Mark J. Shlomchik,†,x David G. -

Inline-Supplementary-Material-6.Pdf
Table S3. Transcriptome analysis page 1 Table S3. Transcriptomic analysis of cultured mutant and wildtype skin fibroblast. Gene Protein name GeneCards annotation OMIM Locus Fold FDR PATIENTS CONTROLS - change (n=2) (n=4) ment apoptosis apoptosis Keratinocyte Keratinocyte inflammation differentiation Neurodevelop cell growth and growth cell FPKM [SD] FPKM [SD] genes up-regulated in patient cells ESRP1 epithelial ESRP1 is an mRNA splicing factor that regulates the *612959, the gene product is an 8:95653301 - present - 0.011 0.3547 0.0038 0.0000 0.0000 splicing formation of epithelial cell-specific isoforms. epithelial cell-type-specific splicing 95719694 absent regulatory Specifically regulates the expression of FGFR2-IIIb, regulator. Mutations in ESRP1 are protein 1 an epithelial cell-specific isoform of FGFR2. Also potentially associated with autosomal regulates the splicing of CD44, CTNND1, ENAH, recessive deafness type 109. three transcripts that undergo changes in splicing during the epithelial-to-mesenchymal transition (EMT). CERS3 ceramide CERS3 is a member of the ceramide synthase family #615023, autosomal recessive 15:100913143 - present - 0.011 0.1844 0.0118 0.0000 0.0000 synthase 3 of genes. The ceramide synthase enzymes regulate congenital ichthyosis type 9, 101085200 absent sphingolipid synthesis by catalyzing the formation characterized by collodian membrane at of ceramides from sphingoid base and acyl-CoA birth, acanthosis, orthohyperkeratosis, substrates. This family member is involved in the fine erythrodermic scales, palmoplantar synthesis of ceramides with ultra-long-chain acyl- hyperlinearity. moieties (ULC-Cers), important to the epidermis in its role in creating a protective barrier from the environment. GJB6 gap junction GJB6 encodes one of the connexin proteins. -

Number 11 November 2020 Atlas of Genetics and Cytogenetics in Oncology and Haematology
Volume 1 - Number 1 May - September 1997 Volume 24 - Number 11 November 2020 Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL INIST-CNRS Scope The Atlas of Genetics and Cytogenetics in Oncology and Haematology is a peer reviewed on-line journal in open access, devoted to genes, cytogenetics, and clinical entities in cancer, and cancer-prone diseases. It is made for and by: clinicians and researchers in cytogenetics, molecular biology, oncology, haematology, and pathology. One main scope of the Atlas is to conjugate the scientific information provided by cytogenetics/molecular genetics to the clinical setting (diagnostics, prognostics and therapeutic design), another is to provide an encyclopedic knowledge in cancer genetics. The Atlas deals with cancer research and genomics. It is at the crossroads of research, virtual medical university (university and post-university e-learning), and telemedicine. It contributes to "meta-medicine", this mediation, using information technology, between the increasing amount of knowledge and the individual, having to use the information. Towards a personalized medicine of cancer. It presents structured review articles ("cards") on: 1- Genes, 2- Leukemias, 3- Solid tumors, 4- Cancer-prone diseases, and also 5- "Deep insights": more traditional review articles on the above subjects and on surrounding topics. It also present 6- Case reports in hematology and 7- Educational items in the various related topics for students in Medicine and in Sciences. The Atlas of Genetics -
Downloaded from the Mouse Lysosome Gene Database, Mlgdb
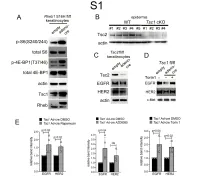
1 Supplemental Figure Legends 2 3 Supplemental Figure S1: Epidermal-specific mTORC1 gain-of-function models show 4 increased mTORC1 activation and down-regulate EGFR and HER2 protein expression in a 5 mTORC1-sensitive manner. (A) Immunoblotting of Rheb1 S16H flox/flox keratinocyte cultures 6 infected with empty or adenoviral cre recombinase for markers of mTORC1 (p-S6, p-4E-BP1) 7 activity. (B) Tsc1 cKO epidermal lysates also show decreased expression of TSC2 by 8 immunoblotting of the same experiment as in Figure 2A. (C) Immunoblotting of Tsc2 flox/flox 9 keratinocyte cultures infected with empty or adenoviral cre recombinase showing decreased EGFR 10 and HER2 protein expression. (D) Expression of EGFR and HER2 was decreased in Tsc1 cre 11 keratinocytes compared to empty controls, and up-regulated in response to Torin1 (1µM, 24 hrs), 12 by immunoblot analyses. Immunoblots are contemporaneous and parallel from the same biological 13 replicate and represent the same experiment as depicted in Figure 7B. (E) Densitometry 14 quantification of representative immunoblot experiments shown in Figures 2E and S1D (r≥3; error 15 bars represent STDEV; p-values by Student’s T-test). 16 17 18 19 20 21 22 23 Supplemental Figure S2: EGFR and HER2 transcription are unchanged with epidermal/ 24 keratinocyte Tsc1 or Rptor loss. Egfr and Her2 mRNA levels in (A) Tsc1 cKO epidermal lysates, 25 (B) Tsc1 cKO keratinocyte lysates and(C) Tsc1 cre keratinocyte lysates are minimally altered 26 compared to their respective controls. (r≥3; error bars represent STDEV; p-values by Student’s T- 27 test). -

Genome-Wide Expression Profiling (With Focus on the Galectin Network)
ANTICANCER RESEARCH 37 : 2275-2288 (2017) doi:10.21873/anticanres.11565 Genome-wide Expression Profiling (with Focus on the Galectin Network) in Tumor, Transition Zone and Normal Tissue of Head and Neck Cancer: Marked Differences Between Individual Patients and the Site of Specimen Origin VERONIKA ZIVICOVA 1,2* , PETR BROZ 3* , ZDENEK FIK 1,2 , ALZBETA MIFKOVA 1,2 , JAN PLZAK 2, ZDENEK CADA 2, HERBERT KALTNER 4, JANA FIALOVA KUCEROVA 3, HANS-JOACHIM GABIUS 4 and KAREL SMETANA JR. 1,5 1Institute of Anatomy, and 2Department of Otorhinolaryngology, Head and Neck Surgery, First Faculty of Medicine, Charles University, Prague, Czech Republic; 3Institute of Applied Biotechnologies, Prague, Czech Republic; 4Institute of Physiological Chemistry, Faculty of Veterinary Medicine, Ludwig Maximilian University, Munich, Germany; 5BIOCEV, First Faculty of Medicine, Charles University, Vestec, Czech Republic Abstract. Background/Aim: Expression profiling was Squamous cell carcinoma (SCC) of the head and neck performed to delineate and characterize the impact of continues to be a major clinical challenge (1). This tumor malignancy by comparing tissues from three sites of head and type is characterized by locally aggressive growth, lymph neck cancer of each patient, also determining interindividual node metastasis and limited spread beyond lymph nodes (2). variability. Materials and Methods: Genome-wide analysis was Radical surgery represents the main therapeutic modality but carried out covering the expression of 25,832 genes with can have unfavorable consequences and reduce quality of quantification for each site of seven patients with tonsillar or life. Clinically, reliable independent prognostic factors can oropharyngeal squamous cell carcinoma. Immunohisto- guide decisions on the strategy of therapy for the individual chemical analysis was performed for adhesion/growth- patient. -

UNIVERSITY of CALIFORNIA Los Angeles Computational Tools for The
UNIVERSITY OF CALIFORNIA Los Angeles Computational tools for the analysis of high-throughput genome-scale sequence data A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Molecular Biology by David Adrian Lopez, Jr. 2016 © Copyright by David Adrian Lopez, Jr. 2016 ABSTRACT OF THE DISSERTATION Computational tools for the analysis of high-throughput genome-scale sequence data by David Adrian Lopez, Jr. Doctor of Philosophy in Molecular Biology University of California, Los Angeles, 2016 Professor Matteo Pellegrini, Chair As high-throughput sequence data becomes increasingly used in a variety of fields, there is a growing need for computational tools that facilitate analyzing and interpreting the sequence data to extract biological meaning. To date, several computational tools have been developed to analyze raw and processed sequence data in a number of contexts. However, many of these tools primarily focus on well-studied, reference organisms, and in some cases, such as the visualization of molecular signatures in expression data, there is a scarcity or complete absence of tools. Furthermore, the compendium of genome-scale data in publicly accessible databases can be leveraged to inform new studies. The focus of this dissertation is the development of computational tools and methods to analyze high-throughput genome-scale sequence data, as well as applications in mammalian, algal, and bacterial systems. Chapter 1 introduces the challenges of analyzing high-throughput sequence data. Chapter 2 presents the Signature Visualization Tool (SaVanT), a framework to visualize molecular signatures in user- ii generated expression data on a sample-by-sample basis. This chapter demonstrates that SaVanT can use immune activation signatures to distinguish patients with different types of acute infections (influenza A and bacterial pneumonia), and determine the primary cell types underlying different leukemias (acute myeloid and acute lymphoblastic) and skin disorders. -

Single-Cell Transcriptome Conservation in a Comparative Analysis of Fresh
Mirizio et al. Arthritis Research & Therapy (2020) 22:263 https://doi.org/10.1186/s13075-020-02343-4 RESEARCH ARTICLE Open Access Single-cell transcriptome conservation in a comparative analysis of fresh and cryopreserved human skin tissue: pilot in localized scleroderma Emily Mirizio1, Tracy Tabib2, Xinjun Wang3, Wei Chen3, Christopher Liu1, Robert Lafyatis2, Heidi Jacobe4† and Kathryn S. Torok1,2,5*† Abstract Background: The purpose of this study was to assess variability in cell composition and cell-specific gene expression in the skin of patients with localized scleroderma (LS) utilizing CryoStor® CS10 in comparison to RPMI to produce adequate preservation of tissue samples and cell types of interest for use in large-scale multi-institutional collaborations studying localized scleroderma and other skin disorders. Methods: We performed single-cell RNA sequencing on paired skin biopsy specimens from 3 patients with LS. Each patient with one sample cryopreserved in CryoStor® CS10 and one fresh in RPMI media using 10× Genomics sequencing. Results: Levels of cell viability and yield were comparable between CryoStor® CS10 (frozen) and RPMI (fresh) preserved cells. Furthermore, gene expression between preservation methods was collectively significantly correlated and conserved across all 18 identified cell cluster populations. Conclusion: Comparable cell population and transcript expression yields between CryoStor® CS10 and RPMI preserved cells support the utilization of cryopreserved skin tissue in single-cell analysis. This suggests that employing standardized cryopreservation protocols for the skin tissue will help facilitate multi-site collaborations looking to identify mechanisms of disease in disorders characterized by cutaneous pathology. Keywords: Localized scleroderma, Morphea, Single-cell RNA sequencing, Pediatric rheumatology, Cryopreservation, Transcriptome expression Background transcriptome heterogeneity on a single-cell level. -

Effects of Weaning Age and Maternal Nutrition on Gene Expression of Longissimus Muscle of Beef Steers by Sonia Josefina Moisá D
EFFECTS OF WEANING AGE AND MATERNAL NUTRITION ON GENE EXPRESSION OF LONGISSIMUS MUSCLE OF BEEF STEERS BY SONIA JOSEFINA MOISÁ DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Animal Sciences in the Graduate College of the University of Illinois at Urbana-Champaign, 2015 Urbana, Illinois Doctoral Committee: Associate Professor Juan J. Loor, Chair Professor James K. Drackley Assistant Professor Daniel W. Shike Associate Professor Yuan-Xiang Pan ABSTRACT Adipogenic/lipogenic transcriptional networks regulating intramuscular fat (IMF) deposition in response weaning age and dietary starch level were studied. The longissimus muscle (LM) of beef steers on an early weaning (141 days age) plus high-starch diet (EWS) or a normal weaning (NW, 222 days age) plus starch creep-feed diet (CFS) was biopsied at 0 (EW), 25, 50, 96 (NW), 167, and 222 (pre-slaughter) days. Expression patterns of 35 target genes were studied. From NW through slaughter all steers received the same high-starch diet. In EWS steers the expression of PPARG, other adipogenic (CEBPA, ZFP423) and lipogenic (THRSP, SREBF1, INSIG1) activators, and several enzymes (FASN, SCD, ELOVL6, PCK1, DGAT2) that participate in the process of IMF increased gradually to a peak between 96 and 167 days on treatment. Steers in NW did not achieve similar expression levels even by 222 days on treatment, suggesting a blunted response even when fed a high-starch diet after weaning. In conclusion, high-starch feeding at an early age (EWS) triggers precocious and sustained adipogenesis resulting in greater marbling. In a following study, we wanted to test if the exposure to an increased maternal nutrition during late gestation might result in an early increase in the expression of genes and/or other changes due to epigenetic regulation that leads to adipogenesis and lipogenesis in the offspring’s LM. -

Differential Gene Expression Landscape of Co-Existing Cervical Pre-Cancer Lesions Using RNA-Seq
ORIGINAL RESEARCH ARTICLE published: 26 November 2014 doi: 10.3389/fonc.2014.00339 Differential gene expression landscape of co-existing cervical pre-cancer lesions using RNA-seq Kathryn E. Royse 1, Degui Zhi 2, Michael G. Conner 3, Buffie Clodfelder-Miller 4,Vinodh Srinivasasainagendra2, Laura Kelly Vaughan2, Christine F. Skibola1, David K. Crossman5, Shawn Levy 6 and Sadeep Shrestha1* 1 Department of Epidemiology, University of Alabama at Birmingham, Birmingham, AL, USA 2 Department of Biostatistics, University of Alabama at Birmingham, Birmingham, AL, USA 3 Department of Pathology, University of Alabama at Birmingham, Birmingham, AL, USA 4 Cellular and Molecular Neuropathology Core, University of Alabama at Birmingham, Birmingham, AL, USA 5 Department of Genetics, University of Alabama at Birmingham, Birmingham, AL, USA 6 Hudson Alpha Institute for Biotechnology, Huntsville, AL, USA Edited by: Genetic changes occurring in different stages of pre-cancer lesions reflect causal events Mike Eccles, University of Otago, initiating and promoting the progression to cancer. Co-existing pre-cancerous lesions New Zealand including low- and high-grade squamous intraepithelial lesion (LGSIL and HGSIL), and adja- Reviewed by: Cristin Gregor Print, University of cent “normal” cervical epithelium from six formalin-fixed paraffin-embedded samples were Auckland, New Zealand selected. Tissues from these 18 samples were isolated using laser-capture microdissec- Aniruddha Chatterjee, University of tion, RNA was extracted and sequenced. RNA-sequencing generated 2.4 billion raw reads Otago, New Zealand in 18 samples, of which ~50.1% mapped to known and annotated genes in the human *Correspondence: genome. There were 40 genes up-regulated and 3 down-regulated (normal to LGSIL) in at Sadeep Shrestha, Department of Epidemiology, University of Alabama least one-third of the sample pairs (same direction and FDR p < 0.05) including S100A7 at Birmingham, 1665 University Blvd, and KLK6.