Catheter Ablation for Atrial Fibrillation - Project ID: CRDT0913
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Incidence of Post Cross Clamp Ventricular Fibrillation in Isolated Coronary Artery Bypass Surgery Using Del Nido Cardioplegia and Conventional Blood Cardioplegia
Jemds.com Original Research Article Incidence of Post Cross Clamp Ventricular Fibrillation in Isolated Coronary Artery Bypass Surgery Using del Nido Cardioplegia and Conventional Blood Cardioplegia Biju Kambil Thyagarajan1, Anandakuttan Sreenivasan2, Ravikrishnan Jayakumar3, Ratish Radhakrishnan4 1, 2, 3, 4 Department of Cardiovascular and Thoracic Surgery, Government T D Medical College, Alappuzha, Kerala, India. ABSTRACT BACKGROUND The cardiac surgical procedures and surgical outcomes witnessed a dramatic Corresponding Author: improvement with the introduction of cardiopulmonary bypass and cardioplegia Dr. Anandakuttan Sreenivasan, techniques. Ventricular fibrillation immediately after removal of aortic cross clamp is Department of Cardiovascular and an energy consuming process leading to myocardial injury in an already energy Thoracic Surgery, Government T D Medical College, Alappuzha, Kerala, India, depleted heart. Electrical cardioversion, which itself causes myocardial injury, is E-mail: [email protected] required to regain normal rhythm. Prevention of ventricular fibrillation is important in preventing myocardial injury. We retrospectively analysed the incidence of post DOI: 10.14260/jemds/2020/797 cross clamp ventricular fibrillation requiring electrical defibrillation in isolated coronary artery bypass surgery using del Nido cardioplegia and conventional blood How to Cite This Article: cardioplegia. Thyagarajan BK, Sreenivasan A, Jayakumar R, et al. Incidence of post cross clamp METHODS ventricular fibrillation in isolated coronary -

Cardiac Arrhythmia and Catheter Ablation UK
Media backgrounder Cardiac Arrhythmia and Catheter Ablation Technique Cardiac arrhythmia – when the heart falls out of rhythm Cardiac arrhythmias (CA) represent a group of conditions with an abnormal heart rhythm or heart rate. This may involve the heart beating too fast (over 100 bpm), too slow (less than 60 bpm) or irregularly.1 Arrhythmias are often caused by problems with the electrical system that regulates the steady heartbeat and can originate in the lower chambers of the heart (ventricles) or in the upper chambers (atria).2 There are various types of arrhythmia; the most common forms include atrial fibrillation (AF), atrial flutter and atrioventricular nodal re-entrant tachycardia. Noticeable warning symptoms include fluttering in the chest, shortness of breath, fatigue, chest pain, dizziness or fainting.3 Many people experience irregular heartbeats at some point in their lives. Most of the time they are harmless, especially when not associated with other heart conditions. However, some arrhythmias can be serious and even life-threatening if left untreated. For example, atrial fibrillation is the most common cardiac arrhythmia and an independent contributor to mortality, morbidity and impaired quality of life.4,5 According to data from the long-term, ongoing Framingham heart study, AF is associated with a 1.5- to 1.9-fold higher risk of death. This is in part attributed to the strong association between AF and thromboembolic events (where the blood in the heart can clot).6 Overall, AF is estimated to be responsible for approximately 15 percent of all strokes7,8,9 and 20 percent of all ischemic strokes.10 Cardiac arrhythmia – a growing global burden • Anyone can develop arrhythmias, even young adults without previous heart problems. -

Radiofrequency Catheter Ablation of Persistent Atrial Fibrillation with Myotonic Dystrophy and Achalasia-Like Esophageal Dilatation Bong Joon Kim
CASE REPORTS International Journal of Arrhythmia 2017;18(4):205-208 doi: http://dx.doi.org/10.18501/arrhythmia.2017.031 Radiofrequency Catheter Ablation of Persistent Atrial Fibrillation with Myotonic Dystrophy and Achalasia-like Esophageal Dilatation Bong Joon Kim Bong-Joon Kim, MD; Tae-Joon Cha, ABSTRACT MD, PhD, FHRS Myotonic dystrophy, a multi-systemic disease with cardiac involve- Division of Cardiology, Kosin University Gospel ment, is the most common inherited neuromuscular disease. Here, Hospital, Busan, Republic of Korea we report the results of radiofrequency catheter ablation of persis- tent atrial fibrillation in a patient with myotonic dystrophy and acha- Received: September 25, 2017 Accepted: September 29, 2017 lasia-like esophageal dilatation. Correspondence: Tae-Joon Cha, MD, PhD, FHRS Division of Cardiology, Kosin University Gospel Hospital, 262, Gamchen-Ro, Seo-Gu, Busan 49267, Republic of Korea Tel: +82-51-990-6105, Fax: +82-51-990-3047 E-mail: [email protected] Copyright © 2017 The Official Journal of Korean Heart Rhythm Society Editorial Board and EUM & Communications Key Words: ■Atrial Fibrillation ■Myotonic Dystrophy Introduction Case Myotonic dystrophy (dystrophia myotonica [DM]) is an On March 7, 2009, a 46-year-old man presented with autosomal-dominant, multi-systemic disease for which the clinical palpitation that had persisted for several years. He had undergone presentation includes myotonia, muscular dystrophy, cardiac DC cardioversion for AF 8 months prior. He did not have a involvement, posterior iridescent cataracts, and endocrine history of hypertension, diabetes mellitus, dyslipidemia, or disorders.1 The incidence of myotonic dystrophy type 1 (DM1) ischemic heart disease; however, he had a stroke 4 years prior. -
Computing in Cardiology
COMPUTING IN CARDIOLOGY September 13-16, 2020 Rimini, Italy Table of Contents Sponsors 3 Welcome to CinC@Rimini in 2020! 5 Board of Directors 7 Local Organizing Committee 8 Letter from the President 9 Welcome to Brno for CinC 2021 10 Maps 11 General Map of Rimini 11 Transportation, Hotels and Practical Information 14 Transportation 14 By air 14 By car 14 By train 14 Local Transportation in Rimini 15 By bus 15 By bike 15 Practical Information 16 Climate 16 Money/currency 16 Emergency phone numbers 16 Electric standards 16 Language 17 Time Zones 17 Mobile Phones 17 Safety and Security 17 COVID-19 emergency – Main general rules in Emilia - Romagna 17 COVID-19 emergency – Safety rules and procedures at Palacongressi 18 Internet Access 19 Computing in Cardiology 2020 1 Meals 20 Accompanying Persons (Guests) 20 Conference Information 21 General Information 21 Sunday Symposium 21 Programme outline 21 Conference site 22 Monday Social Program 23 Activist program 23 Passivist program 24 For Authors and Speakers 25 Oral presentations 25 IN PERSON oral presentations 25 REMOTE oral presentations 26 Q&A during oral presentations 26 Poster presentations 26 IN PERSON poster session 26 REMOTE poster session 27 Rosanna Degani Young Investigator Award 28 Clinical Needs Translational (CTA) Award 28 PhysioNet/Computing in Cardiology Challenge 2020 28 Maastricht Simulation Award (MSA) 29 Deadlines 29 Manuscripts 29 Scientific Program Details 31 Program Overview 2 Computing in Cardiology 2020 Sponsors Computing in Cardiology 2020 is supported by several institutions, companies and academic partnerships. The Local Organizing Committee would like to thank the following partners: Computing in Cardiology 2020 3 4 Computing in Cardiology 2020 Welcome to CinC@Rimini in 2020! Dear Colleagues and Friends, On behalf of the Local Organizing Committee, we warmly welcome you to Computing in Cardiology 2020. -

Clinical Guideline Experimental Or Investigational Services
Clinical Guideline Guideline Number: CG012, Ver. 6 Experimental or Investigational Services Disclaimer Clinical guidelines are developed and adopted to establish evidence-based clinical criteria for utilization management decisions. Oscar may delegate utilization management decisions of certain services to third-party delegates, who may develop and adopt their own clinical criteria. Clinical guidelines are applicable to certain plans. Clinical guidelines are applicable to members enrolled in Medicare Advantage plans only if there are no criteria established for the specified service in a Centers for Medicare & Medicaid Services (CMS) national coverage determination (NCD) or local coverage determination (LCD) on the date of a prior authorization request. Services are subject to the terms, conditions, limitations of a member’s policy and applicable state and federal law. Please reference the member’s policy documents (e.g., Certificate/Evidence of Coverage, Schedule of Benefits) or contact Oscar at 855-672-2755 to confirm coverage and benefit conditions. Summary The services referenced in this Clinical Guideline are considered experimental or investigational and are therefore not covered by Oscar. The services referenced in this Clinical Guideline may not be all- inclusive. Specific benefit plan documents (e.g., Certificate of Coverage, Schedule of Benefits) and federal or state mandated health benefits and laws take precedence over this Clinical Guideline. A service considered experimental or investigational when its safety and efficacy has been established. They may have outcomes that are inferior to standard medical treatment, for which long-term clinical utility has been established. To determine whether a service, device, treatment or procedure has proven safety and efficacy, the available reliable evidence is reviewed, which may include but is not limited to (listed in order of decreasing reliability): 1. -

CT Coronary Angiography for Detection of Coronary Artery Obstruction, Without the Need for Further Imaging Studies Or Additional Radiation Exposure
IAEA Department of Technical Cooperation And Nuclear Medicine Section RAS 6/063 Strengthening the Application of Nuclear Medicine in the Management of Cardiovascular Diseases Cardiac Imaging CT and MR Prof Lin Tun Tun Head of Department of Radiology University of Medicine (1) Yangon General Hospital CARDIAC CT Advances in CT Technology • Increased image quality • Improvements in hardware and software such as refined image reconstruction methods. • lower radiation exposure of cardiac CT especially for coronary CTA INDICATIONS FOR CARDIAC CT • Chest pain with intermediate pretest probability of CAD • Acute coronary syndrome with intermediate pretest probability of CAD (no ECG changes and negative serial enzymes negative) Evaluation of bypass grafts and coronary anatomy • Evaluation of complex congenital heart disease (anomalies of coronary circulation, great vessels, and cardiac chambers and valves) • Evaluation of cardiac masses or pericardial conditions • Evaluation of pulmonary vein anatomy before radiofrequency ablation, coronary vein • Evaluation of suspected aortic dissection, aortic aneurysm, or pulmonary embolism. History • EBCT – mid 1990 – 1.5 to 3mm slice thickness • 4 MSCT -2000- 1mm s thickness (30 heart beats minimum over all image-acquisition time) • 4-16-64 MSCT - 2004 -0.5 to 0.75 mm (4-8 heart beats) EBCT- Electron Beam CT Evolution of CT - 40 years ‘72. …85…… 89…..91 …….95 ……98.99…01..02..04…05…2006----2012 1st CT Slip Ring Spiral Twin ub sec ½ sec 64 DSCT 256 / ……640/ CT detector MSCT /FPD DSCT/DECT 0.33sec/ritation Multidetector, Multisource CT New detector technologies Data Handling, Dose management Dual source CT 2005- Dual Source CT 2 X-ray sources and 2 detectors Faster than every beating heart DSCT became available around the year 2005, with 2 64 simultaneously acquired slices and a rotation time of 33 milliseconds and More recently, with 2 128 simultaneously acquired slices and a gantry rotation time of 0.28 seconds. -

Pulmonary Vein Isolation Induces Changes in Vectorcardiogram P-Wave Loops
Pulmonary Vein Isolation Induces Changes in Vectorcardiogram P-wave Loops Nuria Ortigosa1, Oscar´ Cano2, Frida Sandberg3 1 I.U. Matematica´ Pura y Aplicada, Universitat Politecnica` de Valencia,` Spain 2 Servicio de Cardiolog´ıa, Hospital Universitari i Politecnic` La Fe. Valencia, Spain 3 Department of Biomedical Engineering, Lund University, Sweden Abstract ful AF ablation has been the durable PVI [6] and it is there- fore desirable to study changes induced by the procedure Pulmonary vein isolation (PVI) is considered a stan- that may be linked to durability of the PVI. dard treatment of paroxysmal atrial fibrillation aiming to Previous studies have analysed how PVI changes the restore and maintain sinus rhythm. The purpose of this surface ECG signal by decreasing the P-wave duration [7, study is to analyse how the PVI treatment affects the elec- 8], and P-wave amplitude and duration dispersion [9–11]. trical conduction pattern in the atria. We have compared In this study we propose to analyse the vectorcardio- the morphology of P wave loops extracted from the vector- gram (VCG) to assess how PVI affects the electrical con- cardiogram (VCG) before and after PVI. duction pattern in the atria. Based on the 12-lead surface Ten patients suffering from paroxysmal atrial fibrillation ECG of patients with paroxysmal AF in sinus rhythm, we who underwent PVI were included in the study. All patients have extracted the VCG, and then compared the morphol- were in sinus rhythm before as well as after the procedure. ogy of P-wave loops before and after PVI to reveal changes Vectorcardiogram was obtained using the Kors matrix and induced by the procedure. -
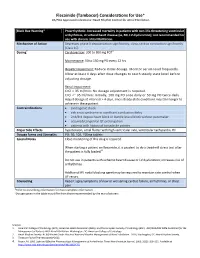
Flecainide Considerations For
Flecainide (Tambocor) Considerations for Use* US/FDA Approved Indications: Heart Rhythm Control for Atrial Fibrillation Black Box Warning* Proarrhythmic. Increased mortality in patients with non-life-threatening ventricular arrhythmias, structural heart disease (ie, MI, LV dysfunction); not recommended for use with chronic atrial fibrillation. Mechanism of Action Depresses phase 0 depolarization significantly, slows cardiac conduction significantly (Class 1C). Dosing† Cardioversion: 200 to 300 mg PO‡1 Maintenance: 50 to 150 mg PO every 12 hrs Hepatic Impairment: Reduce initial dosage. Monitor serum level frequently. Allow at least 4 days after dose changes to reach steady state level before adjusting dosage. Renal Impairment: CrCl > 35 ml/min: No dosage adjustment is required. CrCl <= 35 ml/min: Initially, 100 mg PO once daily or 50 mg PO twice daily. Adjust dosage at intervals > 4 days, since steady-state conditions may take longer to achieve in these patient Contraindications cardiogenic shock sick sinus syndrome or significant conduction delay 2nd/3rd degree heart block or bundle brand block without pacemaker acquired/congenital QT prolongation patients with history of torsade de pointes Major Side Effects hypotension, atrial flutter with high ventricular rate, ventricular tachycardia, HF Dosage forms and Strengths PO: 50, 100, 150mg tablets Special Notes Close monitoring of this drug is required. When starting a patient on flecainide, it is prudent to do a treadmill stress test after the patient is fully loaded.4 Do not use in patients with ischemic heart disease or LV dysfunction; increases risk of arrhythmias. Additional AV nodal blocking agent may be required to maintain rate control when AF recurs. -

Coronary Sinus Cryoablation of Ventricular Tachycardia After Failed Radiofrequency Ablation
www.symbiosisonline.org Symbiosis www.symbiosisonlinepublishing.com Case Report Journal of Clinical Trials in Cardiology Open Access Coronary Sinus Cryoablation of Ventricular Tachycardia after Failed Radiofrequency Ablation Jiménez-Fernández M*, Macías- Ruiz R, Álvarez-López M, and Tercedor- Sánchez L Virgen de las Nieves University Hospital, Granada, Spain Received: March 11, 2014; Accepted: September 10, 2014; Published: September 25, 2014 *Corresponding author: Miriam Jiménez-Fernández, Virgen de las Nieves University Hospital, Granada, Spain, E-mail: [email protected] Abstract We present a case of Great Cardiac Vein (GCV) cryoablation in order to suppress idiopathic epicardial Ventricular Tachycardia (VT) after failed Radiofrequency (RF) ablation via the epicardium and GCV. RF ablation is the technique of choice for the treatment of Ventricular Arrhythmias (VA). However, this procedure has its limitations when delivered inside the distal cardiac veins. Cryoablation is a good alternative in patients where RF cannot be used because of risk of high impedance. Keywords: Percutaneous epicardial ablation; Ventricular tachycardia; Radiofrequency; Catheter ablation; Cryoablation; Coronary sinus; High impedance; Cryoenergy; Great cardiac vein; Mapping; Electroanatomic mapping system; 12-lead surface electrocardiogram Case Report tachycardia, but the delivery of RF had to be interrupted with a maximal energy of 15 W, as the impedance rise exceeded the A 56-year-old female patient presented in emergency room referring shortness of breath and chest pain. ECG showed we decided to change to a 6 mm tip cryoablation catheter that was sustained monomorphic VT at 160 beats per minute with right ablesafety to limit reach (>300 the programmed Ω) (Figure 1C). temperature In order to of solve -80°C this suppressing problem, bundle branch block and right inferior axis morphology (Figure the VT with no further recurrences. -

Discharge Advice After Atrial Fibrillation Ablation
Oxford University Hospitals NHS Trust Oxford Heart Centre Discharge advice after Atrial Fibrillation ablation Information for patients page 2 This booklet contains important advice about discharge after your Atrial Fibrillation (AF) ablation. It contains information about what to do when you get home. Contents 1. Discharge summary 4 Follow-up 4 Transport 4 2. What to do when you get home 5 Puncture site care 5 Bleeding 6 Sedation/General Anaesthetic 6 After the catheter ablation 6 Recurrence of AF symptoms - what to do 6 Driving 7 Return to work 8 3. Medication 8 4. How to contact us 9 5. Further information 10 6. Message for doctor reviewing this patient 10 page 3 1. Discharge summary Your Consultant at the John Radcliffe Hospital is: ………………………….…............................................................…….. Follow-up You will be sent an appointment for follow-up in the Arrhythmia clinic. (This appointment will be sent in the post. If you do not receive a date for an appointment within 8 weeks, please call the John Radcliffe Hospital and ask to speak to the secretary of your Consultant. Follow-up appointments are currently planned approximately 3 to 4 months after your procedure.) Transport to your outpatient appointments If you have difficulty getting to your outpatient appointments your GP surgery may have the phone numbers of voluntary transport schemes which operate at subsidised rates. A directory of these services is available at www.oxonrcc.org.uk for residents of the Oxfordshire area. page 4 2. What to do when you get home When you are discharged home, you should have a quiet few days resting to recover from your procedure. -

Resuscitation and Defibrillation
AARC GUIDELINE: RESUSCITATION AND DEFIBRILLATION AARC Clinical Practice Guideline Resuscitation and Defibrillation in the Health Care Setting— 2004 Revision & Update RAD 1.0 PROCEDURE: signs, level of consciousness, and blood gas val- Recognition of signs suggesting the possibility ues—included in those conditions are or the presence of cardiopulmonary arrest, initia- 4.1 Airway obstruction—partial or complete tion of resuscitation, and therapeutic use of de- 4.2 Acute myocardial infarction with cardio- fibrillation in adults. dynamic instability 4.3 Life-threatening dysrhythmias RAD 2.0 DESCRIPTION/DEFINITION: 4.4 Hypovolemic shock Resuscitation in the health care setting for the 4.5 Severe infections purpose of this guideline encompasses all care 4.6 Spinal cord or head injury necessary to deal with sudden and often life- 4.7 Drug overdose threatening events affecting the cardiopul- 4.8 Pulmonary edema monary system, and involves the identification, 4.9 Anaphylaxis assessment, and treatment of patients in danger 4.10 Pulmonary embolus of or in frank arrest, including the high-risk de- 4.11 Smoke inhalation livery patient. This includes (1) alerting the re- 4.12 Defibrillation is indicated when cardiac suscitation team and the managing physician; (2) arrest results in or is due to ventricular fibril- using adjunctive equipment and special tech- lation.1-5 niques for establishing, maintaining, and moni- 4.13 Pulseless ventricular tachycardia toring effective ventilation and circulation; (3) monitoring the electrocardiograph and recogniz- -

Atrial Fibrillation Ablation
Atrial Fibrillation Ablation Atrial Fibrillation Ablation This handout will help you learn about atrial fibrillation ablation, also called pulmonary vein isolation. © Hamilton Health Sciences, 2008 PD 6062 – 02/2012 dpc/pted/LrgBk/AtrialFibrillationAblation-trh.doc dt/February 27, 2012 2 15 Atrial Fibrillation Ablation Atrial Fibrillation Ablation How does the heart work? Notes: To understand atrial fibrillation, you need to know how the heart’s electrical system works. __________________________________________________________ The sinoatrial node (SA node) is a natural pacemaker. It starts the __________________________________________________________ electrical signal that travels across the upper 2 chambers or atria of the heart to the atrioventricular node (AV node). __________________________________________________________ The AV node transfers the electrical signal from the upper part of the heart to the lower 2 pumping chambers or ventricles. The bundle branches are __________________________________________________________ specialized tissue that help send electrical impulses through the ventricles. This makes a normal heart beat, called normal sinus rhythm. __________________________________________________________ __________________________________________________________ __________________________________________________________ SA node __________________________________________________________ Bundle branches __________________________________________________________ AV node __________________________________________________________