Pulmonary Vein Isolation Induces Changes in Vectorcardiogram P-Wave Loops
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardiac Arrhythmia and Catheter Ablation UK
Media backgrounder Cardiac Arrhythmia and Catheter Ablation Technique Cardiac arrhythmia – when the heart falls out of rhythm Cardiac arrhythmias (CA) represent a group of conditions with an abnormal heart rhythm or heart rate. This may involve the heart beating too fast (over 100 bpm), too slow (less than 60 bpm) or irregularly.1 Arrhythmias are often caused by problems with the electrical system that regulates the steady heartbeat and can originate in the lower chambers of the heart (ventricles) or in the upper chambers (atria).2 There are various types of arrhythmia; the most common forms include atrial fibrillation (AF), atrial flutter and atrioventricular nodal re-entrant tachycardia. Noticeable warning symptoms include fluttering in the chest, shortness of breath, fatigue, chest pain, dizziness or fainting.3 Many people experience irregular heartbeats at some point in their lives. Most of the time they are harmless, especially when not associated with other heart conditions. However, some arrhythmias can be serious and even life-threatening if left untreated. For example, atrial fibrillation is the most common cardiac arrhythmia and an independent contributor to mortality, morbidity and impaired quality of life.4,5 According to data from the long-term, ongoing Framingham heart study, AF is associated with a 1.5- to 1.9-fold higher risk of death. This is in part attributed to the strong association between AF and thromboembolic events (where the blood in the heart can clot).6 Overall, AF is estimated to be responsible for approximately 15 percent of all strokes7,8,9 and 20 percent of all ischemic strokes.10 Cardiac arrhythmia – a growing global burden • Anyone can develop arrhythmias, even young adults without previous heart problems. -

Radiofrequency Catheter Ablation of Persistent Atrial Fibrillation with Myotonic Dystrophy and Achalasia-Like Esophageal Dilatation Bong Joon Kim
CASE REPORTS International Journal of Arrhythmia 2017;18(4):205-208 doi: http://dx.doi.org/10.18501/arrhythmia.2017.031 Radiofrequency Catheter Ablation of Persistent Atrial Fibrillation with Myotonic Dystrophy and Achalasia-like Esophageal Dilatation Bong Joon Kim Bong-Joon Kim, MD; Tae-Joon Cha, ABSTRACT MD, PhD, FHRS Myotonic dystrophy, a multi-systemic disease with cardiac involve- Division of Cardiology, Kosin University Gospel ment, is the most common inherited neuromuscular disease. Here, Hospital, Busan, Republic of Korea we report the results of radiofrequency catheter ablation of persis- tent atrial fibrillation in a patient with myotonic dystrophy and acha- Received: September 25, 2017 Accepted: September 29, 2017 lasia-like esophageal dilatation. Correspondence: Tae-Joon Cha, MD, PhD, FHRS Division of Cardiology, Kosin University Gospel Hospital, 262, Gamchen-Ro, Seo-Gu, Busan 49267, Republic of Korea Tel: +82-51-990-6105, Fax: +82-51-990-3047 E-mail: [email protected] Copyright © 2017 The Official Journal of Korean Heart Rhythm Society Editorial Board and EUM & Communications Key Words: ■Atrial Fibrillation ■Myotonic Dystrophy Introduction Case Myotonic dystrophy (dystrophia myotonica [DM]) is an On March 7, 2009, a 46-year-old man presented with autosomal-dominant, multi-systemic disease for which the clinical palpitation that had persisted for several years. He had undergone presentation includes myotonia, muscular dystrophy, cardiac DC cardioversion for AF 8 months prior. He did not have a involvement, posterior iridescent cataracts, and endocrine history of hypertension, diabetes mellitus, dyslipidemia, or disorders.1 The incidence of myotonic dystrophy type 1 (DM1) ischemic heart disease; however, he had a stroke 4 years prior. -

CT Coronary Angiography for Detection of Coronary Artery Obstruction, Without the Need for Further Imaging Studies Or Additional Radiation Exposure
IAEA Department of Technical Cooperation And Nuclear Medicine Section RAS 6/063 Strengthening the Application of Nuclear Medicine in the Management of Cardiovascular Diseases Cardiac Imaging CT and MR Prof Lin Tun Tun Head of Department of Radiology University of Medicine (1) Yangon General Hospital CARDIAC CT Advances in CT Technology • Increased image quality • Improvements in hardware and software such as refined image reconstruction methods. • lower radiation exposure of cardiac CT especially for coronary CTA INDICATIONS FOR CARDIAC CT • Chest pain with intermediate pretest probability of CAD • Acute coronary syndrome with intermediate pretest probability of CAD (no ECG changes and negative serial enzymes negative) Evaluation of bypass grafts and coronary anatomy • Evaluation of complex congenital heart disease (anomalies of coronary circulation, great vessels, and cardiac chambers and valves) • Evaluation of cardiac masses or pericardial conditions • Evaluation of pulmonary vein anatomy before radiofrequency ablation, coronary vein • Evaluation of suspected aortic dissection, aortic aneurysm, or pulmonary embolism. History • EBCT – mid 1990 – 1.5 to 3mm slice thickness • 4 MSCT -2000- 1mm s thickness (30 heart beats minimum over all image-acquisition time) • 4-16-64 MSCT - 2004 -0.5 to 0.75 mm (4-8 heart beats) EBCT- Electron Beam CT Evolution of CT - 40 years ‘72. …85…… 89…..91 …….95 ……98.99…01..02..04…05…2006----2012 1st CT Slip Ring Spiral Twin ub sec ½ sec 64 DSCT 256 / ……640/ CT detector MSCT /FPD DSCT/DECT 0.33sec/ritation Multidetector, Multisource CT New detector technologies Data Handling, Dose management Dual source CT 2005- Dual Source CT 2 X-ray sources and 2 detectors Faster than every beating heart DSCT became available around the year 2005, with 2 64 simultaneously acquired slices and a rotation time of 33 milliseconds and More recently, with 2 128 simultaneously acquired slices and a gantry rotation time of 0.28 seconds. -

Discharge Advice After Atrial Fibrillation Ablation
Oxford University Hospitals NHS Trust Oxford Heart Centre Discharge advice after Atrial Fibrillation ablation Information for patients page 2 This booklet contains important advice about discharge after your Atrial Fibrillation (AF) ablation. It contains information about what to do when you get home. Contents 1. Discharge summary 4 Follow-up 4 Transport 4 2. What to do when you get home 5 Puncture site care 5 Bleeding 6 Sedation/General Anaesthetic 6 After the catheter ablation 6 Recurrence of AF symptoms - what to do 6 Driving 7 Return to work 8 3. Medication 8 4. How to contact us 9 5. Further information 10 6. Message for doctor reviewing this patient 10 page 3 1. Discharge summary Your Consultant at the John Radcliffe Hospital is: ………………………….…............................................................…….. Follow-up You will be sent an appointment for follow-up in the Arrhythmia clinic. (This appointment will be sent in the post. If you do not receive a date for an appointment within 8 weeks, please call the John Radcliffe Hospital and ask to speak to the secretary of your Consultant. Follow-up appointments are currently planned approximately 3 to 4 months after your procedure.) Transport to your outpatient appointments If you have difficulty getting to your outpatient appointments your GP surgery may have the phone numbers of voluntary transport schemes which operate at subsidised rates. A directory of these services is available at www.oxonrcc.org.uk for residents of the Oxfordshire area. page 4 2. What to do when you get home When you are discharged home, you should have a quiet few days resting to recover from your procedure. -

Atrial Fibrillation Ablation
Atrial Fibrillation Ablation Atrial Fibrillation Ablation This handout will help you learn about atrial fibrillation ablation, also called pulmonary vein isolation. © Hamilton Health Sciences, 2008 PD 6062 – 02/2012 dpc/pted/LrgBk/AtrialFibrillationAblation-trh.doc dt/February 27, 2012 2 15 Atrial Fibrillation Ablation Atrial Fibrillation Ablation How does the heart work? Notes: To understand atrial fibrillation, you need to know how the heart’s electrical system works. __________________________________________________________ The sinoatrial node (SA node) is a natural pacemaker. It starts the __________________________________________________________ electrical signal that travels across the upper 2 chambers or atria of the heart to the atrioventricular node (AV node). __________________________________________________________ The AV node transfers the electrical signal from the upper part of the heart to the lower 2 pumping chambers or ventricles. The bundle branches are __________________________________________________________ specialized tissue that help send electrical impulses through the ventricles. This makes a normal heart beat, called normal sinus rhythm. __________________________________________________________ __________________________________________________________ __________________________________________________________ SA node __________________________________________________________ Bundle branches __________________________________________________________ AV node __________________________________________________________ -
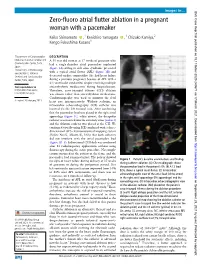
Zero-Fluoro Atrial Flutter Ablation in a Pregnant Woman with a Pacemaker
Images in… BMJ Case Rep: first published as 10.1136/bcr-2020-240671 on 17 March 2021. Downloaded from Zero- fluoro atrial flutter ablation in a pregnant woman with a pacemaker Keiko Shimamoto ,1 Kenichiro Yamagata ,1 Chizuko Kamiya,2 Kengo Fukushima Kusano1 1Department of Cardiovascular DESCRIPTION Medicine, National Cerebral and A- 33- year old woman at 17 weeks of gestation who Cardiovascular Center, Suita, had a single- chamber atrial pacemaker implanted Japan (figure 1A) owing to sick sinus syndrome presented 2Department of Perinatology with a typical atrial flutter (AFL) (figure 1B) and and Obstetrics, National Cerebral and Cardiovascular decreased cardiac contractility. She had heart failure Center, Suita, Japan during a previous pregnancy because of AFL with a 2:1 ventricular conduction despite receiving multiple Correspondence to antiarrhythmic medications during hospitalisation. Dr Kenichiro Yamagata; Therefore, cavo- tricuspid isthmus (CTI) ablation look. tky@ ncvc. go. jp was chosen rather than antiarrhythmic medications. Cardiotocography was used to monitor the fetal Accepted 26 February 2021 heart rate intraoperatively. Without sedation, an intracardiac echocardiography (ICE) catheter was inserted via the left femoral vein. After confirming that the pacemaker lead was placed in the right atrial appendage (figure 1C, white arrow), the decapolar catheter was inserted into the coronary sinus (video 1) and the ablation catheter was placed at the CTI. We confirmed visually using ICE combined with a three- dimensional (3D) electroanatomical mapping system (EnSite NavX, Abbott, IL, USA) that both catheters did not interfere with the atrial pacemaker lead (figure 1D–F). Bidirectional CTI block was confirmed http://casereports.bmj.com/ after 12 radiofrequency applications without using fluoroscopy during the entire procedure. -

2019 HRS/EHRA/APHRS/LAHRS Expert Consensus Statement on Catheter Ablation of Ventricular Arrhythmias
2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias Edmond M. Cronin, MB, BCh, BAO, FHRS, CCDS, CEPS-A (Chair),1 Frank M. Bogun, MD (Vice-Chair),2 Philippe Maury, MD (EHRA Chair),3 Petr Peichl, MD, PhD (EHRA Vice-Chair),4 Minglong Chen, MD, PhD, FHRS (APHRS Chair),5 Narayanan Namboodiri, MBBS, MD (APHRS Vice-Chair),6 Luis Aguinaga, MD, PhD, FESC, FACC (LAHRS Chair),7 Luiz Roberto Leite, MD, PhD, FHRS (LAHRS Vice-Chair),8 Sana M. Al-Khatib, MD, MHS, FHRS, CCDS,9 Elad Anter, MD,10 Antonio Berruezo, MD, PhD,11,* David J. Callans, MD, FHRS, CCDS,12 Mina K. Chung, MD, FHRS,13,† Phillip Cuculich, MD,14 Andre d’Avila, MD, PhD,15,‡ Barbara J. Deal, MD, FACC,16,x Paolo Della Bella, MD,17,* Thomas Deneke, MD, PhD, FHRS,18,* Timm-Michael Dickfeld, MD, PhD, FACC, FHRS,19 Claudio Hadid, MD,20,{ Haris M. Haqqani, MBBS, PhD, FHRS,21,# G. Neal Kay, MD, CCDS,22 Rakesh Latchamsetty, MD, FHRS,2 Francis Marchlinski, MD, FHRS,12 John M. Miller, MD, FHRS,23,† Akihiko Nogami, MD, PhD,24,** Akash R. Patel, MD, FHRS, CEPS-P,25,†† Rajeev Kumar Pathak, MBBS, PhD, FHRS,26,# Luis C. Saenz Morales, MD,27,{ Pasquale Santangeli, MD, PhD,12 John L. Sapp, Jr., MD, FHRS,28 Andrea Sarkozy, MD, PhD, FEHRA,29,* Kyoko Soejima, MD,30,# William G. Stevenson, MD, FHRS,31 Usha B. Tedrow, MD, MS, FHRS,32 Wendy S. Tzou, MD, FHRS,33 Niraj Varma, MD, PhD,13 Katja Zeppenfeld, MD, PhD, FESC, FEHRA34,* Document Reviewers: Samuel J. -

Catheter Ablation for Supra-Ventricular Tachycardia
Catheter Ablation for Supra-Ventricular Tachycardia Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leafl ets please visit www.uhb.nhs.uk/patient-information-leafl ets.htm About this patient information leaflet This patient information leaflet The Heart is about supra-ventricular tachycardia, one of the main heart rhythm problems we treat with catheter ablation procedures. It is one of a series of leaflets that we have produced, written in everyday language that explains what a particular heart rhythm condition is, what its symptoms are, why it occurs and how it is treated. This booklet has been prepared for individuals preparing to undergo one of the procedures described or for individuals looking for more information about these procedures. The information provided within this booklet does not replace the consultation that takes place between the patient and the doctor. Supra-ventricular tachycardia What is supra-ventricular tachycardia? Supra-ventricular tachycardia (SVT) is a condition that results Supra-Ventricular Tachycardia in the heart beating faster than normal. The rapid heart rate produces a sensation of palpitations or fluttering in the chest. It is an abnormal heart rhythm. The heart has an electrical system that controls the rhythm and speed of the heartbeat. An SVT is caused by an extra electrical connection within the smaller, upper chambers of the heart (called atria). This extra connection allows the normal electrical system to run faster and out of control. These episodes or attacks of palpitations can last anywhere from minutes to hours. They are often unpredictable. -

Catheter Ablation for Atrial Fibrillation - Project ID: CRDT0913
Final Topic Refinement Document Catheter Ablation for Atrial Fibrillation - Project ID: CRDT0913 Date: 05/29/2014 Topic: Catheter Ablation for Atrial Fibrillation – Project ID: CRDT0913 EPC: Pacific Northwest EPC AHRQ Task Order Officer: Kim Wittenberg Partner: CMS 1 Final Topic Refinement Document Catheter Ablation for Atrial Fibrillation - Project ID: CRDT0913 Final Topic Refinement Document Key Questions In patients with longstanding persistent atrial fibrillation (AF), persistent AF, or paroxysmal AF (considered separately): Key Question 1. What is the comparative efficacy and effectiveness of AF catheter ablation on short- (6-12 months) and long- (>12 months) term outcomes in the general adult and Medicare populations? Comparisons of interest include: a) Catheter ablation compared with medical therapy b) Comparing ablation using different energy sources Key Question 2. What are the comparative short- and long-term complications and harms (e.g., periprocedural or device-related harms) associated with AF catheter ablation in the general adult and Medicare populations? Comparisons of interest include: a) Catheter ablation compared with medical therapy b) Comparing ablation using different energy sources Key Question 3. Are there modifications of efficacy, effectiveness, or harms of catheter ablation by patient-level characteristics such as age, sex, type of AF, comorbidities, risk for stroke or bleeding events, condition (i.e., patients with significant left ventricular dysfunction/heart failure or patients with significant left atrial enlargement or left ventricular hypertrophy), provider/setting characteristics or technique/approach? Comparisons of interest include: a) Catheter ablation compared with medical therapy b) Comparing ablation using different energy sources 2 Final Topic Refinement Document Catheter Ablation for Atrial Fibrillation - Project ID: CRDT0913 Draft Analytic Framework Figure 1. -

Efficacy and Safety Outcomes of Catheter Ablation of Non-Valvular Atrial Fibrillation in Korean Practice: Analysis of Nationwide Claims Data
ORIGINAL ARTICLES International Journal of Arrhythmia 2018;19(1):14-21 doi: http://dx.doi.org/10.18501/arrhythmia.2018.002 Efficacy and Safety Outcomes of Catheter Ablation of Non-valvular Atrial Fibrillation in Korean Practice: Analysis of Nationwide Claims Data Gi-Hyeon Seo1; Sung-Won Jang, MD, ABSTRACT PhD2; Yong-Seog Oh, MD, PhD2; Man Young Lee, MD, PhD2 ;Tai-Ho Background and Objectives: The aim of this study was to investi- Rho, MD, PhD2 gate the effectiveness and selected adverse events of radiofrequency catheter ablation in patients treated for drug-refractory atrial fibril- 1Health Insurance Review and Assessment Service, Seoul, Korea lation (AF) using claims data provided by the Health Insurance Re- 2Division of Cardiology, Department of Internal view and Assessment Service, which covers all ablation procedures Medicine, College of Medicine, The Catholic conducted in South Korea. University of Korea, Seoul, Korea Subjects and Methods: The study population included patients who underwent catheter ablation for AF between January 2011 and De- cember 2013. A total of 3,850 patients without a previous history of catheter ablation procedure were enrolled. We analyzed 2-year effectiveness of the index ablation. Any redo ablation, electrical car- dioversion, or use of antiarrhythmic drug beyond the blanking pe- riod of 3 months was considered a failure of index ablation. Results: Overall recurrence rate following AF ablation was 61.1%. The recurrence rate was lower in patients under 65 years old than in those over 65 years old (hazard ratio [HR] 0.89; 95% confidence interval [CI], 0.81 to 0.97, P=0.009). -

Pace and Ablate for AF
AF Association Australia Tel: 1800 050 267 or (02) 61084602 info@atrialfi brillation-au.org www.atrialfi brillation-au.org AF A www.atrialfibrillation-au.org Pace and ablate for AF Rate control in atrial fi brillation (AF) (see the undertaken. After ablation the ventricles usually AF-A patient information sheet, Atrial fi brillation) beat at a very slow rate – this is akin to the means using medical treatment to slow the speed situation people can develop spontaneously (or of the pulse without stopping or preventing the even be born with) called complete heart block. rhythm disturbance itself. As with spontaneous heart block an artifi cial permanent pacemaker is required to elevate the In normal (sinus) rhythm there is one heart rate to a level needed for normal day-to-day coordinated contraction of the upper heart activities. Information and Ablate for AF - Patient Pace chambers (atria) per heartbeat. The electrical signal is then conducted through specialised What is a pacemaker? tissue known as the atrioventricular (AV) node (A for atrium, V for ventricle) which electrically A pacemaker is a device implanted under the skin connects the atria to the lower main pumping which stimulates the heart to beat by delivering chambers (ventricles). Conduction of the a small electrical pulse down fl exible wires placed electrical signal through the AV node triggers against the heart wall. The pacemaker is usually contraction of the ventricles approximately one positioned in the upper front of the chest outside fi fth of a second after atrial contraction. It is the the ribcage. The pacing wire/s (lead/s) are contraction of the ventricles which determines passed down to the heart through the veins which the heartbeat and pulse rate. -

Left Atrial Ejection Fraction Assessed by Prior Cardiac CT Predicts Recurrence of Atrial Fibrillation After Pulmonary Vein Isolation
Journal of Clinical Medicine Article Left Atrial Ejection Fraction Assessed by Prior Cardiac CT Predicts Recurrence of Atrial Fibrillation after Pulmonary Vein Isolation Reinhard Kaufmann 1,* , Richard Rezar 2 , Bernhard Strohmer 2 , Bernhard Wernly 2 , Michael Lichtenauer 2, Wolfgang Hitzl 3 , Matthias Meissnitzer 1, Klaus Hergan 1 and Marcel Granitz 1 1 Department of Radiology, University Hospital Salzburg, Paracelsus Medical University of Salzburg, 5020 Salzburg, Austria; [email protected] (M.M.); [email protected] (K.H.); [email protected] (M.G.) 2 Clinic of Internal Medicine II, Department of Cardiology, Paracelsus Medical University of Salzburg, 5020 Salzburg, Austria; [email protected] (R.R.); [email protected] (B.S.); [email protected] (B.W.); [email protected] (M.L.) 3 Research Office (Biostatistics), Paracelsus Medical University of Salzburg, 5020 Salzburg, Austria; [email protected] * Correspondence: [email protected] Abstract: Assuming that atrial fibrillation (AF) is associated with left atrial remodeling and dysfunc- tion, we hypothesize that left atrial and left atrial appendage ejection fractions (LAEF and LAAEF) are useful and may be more sensitive outcome predictors of pulmonary vein isolation (PVI). Fifty patients who underwent PVI at our institution with available pre-interventional cardiac computed tomography (CT) for procedure planning were included in this retrospective study. The patients were separated into two groups by recurrence and non-recurrence of AF and subgroups of paroxysmal Citation: Kaufmann, R.; Rezar, R.; and persistent AF. Semiautomatic volumetric analysis of the left atrium was used to calculate mor- Strohmer, B.; Wernly, B.; Lichtenauer, M.; Hitzl, W.; phological and functional parameters and optimal cut-offs were calculated using the Youden index.