Cardiac Ablation: Types and Outcomes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Incidence of Post Cross Clamp Ventricular Fibrillation in Isolated Coronary Artery Bypass Surgery Using Del Nido Cardioplegia and Conventional Blood Cardioplegia
Jemds.com Original Research Article Incidence of Post Cross Clamp Ventricular Fibrillation in Isolated Coronary Artery Bypass Surgery Using del Nido Cardioplegia and Conventional Blood Cardioplegia Biju Kambil Thyagarajan1, Anandakuttan Sreenivasan2, Ravikrishnan Jayakumar3, Ratish Radhakrishnan4 1, 2, 3, 4 Department of Cardiovascular and Thoracic Surgery, Government T D Medical College, Alappuzha, Kerala, India. ABSTRACT BACKGROUND The cardiac surgical procedures and surgical outcomes witnessed a dramatic Corresponding Author: improvement with the introduction of cardiopulmonary bypass and cardioplegia Dr. Anandakuttan Sreenivasan, techniques. Ventricular fibrillation immediately after removal of aortic cross clamp is Department of Cardiovascular and an energy consuming process leading to myocardial injury in an already energy Thoracic Surgery, Government T D Medical College, Alappuzha, Kerala, India, depleted heart. Electrical cardioversion, which itself causes myocardial injury, is E-mail: [email protected] required to regain normal rhythm. Prevention of ventricular fibrillation is important in preventing myocardial injury. We retrospectively analysed the incidence of post DOI: 10.14260/jemds/2020/797 cross clamp ventricular fibrillation requiring electrical defibrillation in isolated coronary artery bypass surgery using del Nido cardioplegia and conventional blood How to Cite This Article: cardioplegia. Thyagarajan BK, Sreenivasan A, Jayakumar R, et al. Incidence of post cross clamp METHODS ventricular fibrillation in isolated coronary -

Left Anterior Descending Coronary Artery Dissection During Ventricular Tachycardia Ablation – Case Report
CASE REPORTS Left anterior descending coronary artery dissection during ventricular tachycardia ablation – case report KRESIMIR KORDIC, SIME MANOLA, IVAN ZELJKOVIC, IVICA BENKO, NIKOLA PAVLOVIC University Hospital Center Sisters of Charity, Department of Cardiology, Zagreb, Croatia Fascicular left ventricular tachycardia (VT) is the second most frequent idiopathic left VT in the setting of a structurally normal heart. Catheter ablation is curative in most patients with low complication rates. We report a case of ostial left anterior descending coronary artery (LAD) occlusion during fascicular ventricular tachycardia ablation. Dissection was the most likely cause of LAD obstruction. To the authors’ best knowledge, this is the first case reporting selective LAD dissection during electrophysiology study with no left main coronary artery (LMCA) affection. Key words: ventricular tachycardia, electrophysiology, radiofrequency catheter ablation, ST elevation myocardial infarction, percutaneous coronary intervention. INTRODUCTION An electrocardiogram following cardioversion showed normal sinus rhythm, without preexcitation Fascicular left ventricular tachycardia (VT) is or conduction abnormalities. There was no structural the second most frequent idiopathic left VT, after heart disease found on transthoracic echocardio- left ventricular outflow tract VT, occurring in the graphy. setting of a structurally normal heart [1]. An electrophysiology study was performed According to current ESC guidelines [2], using right femoral approach. During the tachy- catheter ablation is curative in most patients with cardia, His was activated after the ventricular VT without overt structural heart disease, with low activation and tachycardia could be entrained from complication rates (around 3%) [3]. Complications atrium and the ventricle. Based on these findings, include access site vascular complications, thrombo- the diagnosis of fascicular ventricular tachycardia embolism, atrioventricular block, myocardial per- was established. -

Late Presentation of Constrictive Pericarditis After Limited Epicardial Ablation for Inappropriate Sinus Tachycardia
Late presentation of constrictive pericarditis after limited epicardial ablation for inappropriate sinus tachycardia Adam Oesterle, MD,* Amita Singh, MD,* Husam Balkhy, MD,* Aliya N. Husain, MD,† Deborah Moyer, APN,* Roderick Tung, MD, FHRS,* Hemal M. Nayak, MD, FHRS* From the *Center for Arrhythmia Care, Heart and Vascular Center, The University of Chicago Medicine, Chicago, Illinois, and †Department of Pathology, The University of Chicago Medicine, Chicago, Illinois. Introduction Biosense-Webster Thermocool SF catheter (Diamond Bar, CA) was delivered in the endocardium. The ablation catheter The number of radiofrequency catheter ablation (RFA) and the angioplasty balloon were both removed and the procedures performed in the epicardial space is increasing.1 ablation catheter was inserted into the epicardial space Major acute complications (primarily pericardial bleeding) through the deflectable sheath, and 5 focal lesions were and delayed complications have been reported in 5% and 2% – delivered in the epicardium overlying the sinus node. At the of cases, respectively.2 4 To the best of our knowledge, a end of the procedure his heart rate decreased from 140 to 70 single case of constrictive pericarditis after multiple epicar- beats per minute. Kenalog (1 mg/kg) was injected into the dial ablations for ventricular tachycardia has been reported.5 pericardial space and the epicardial sheath was removed We describe a late presentation of constrictive pericarditis immediately after the procedure. The fluid was serous, that occurred after a single percutaneous epicardial without any evidence of bleeding, throughout the case. procedure with limited ablation for inappropriate sinus Twelve hours after the procedure, the patient developed tachycardia. pleuritic chest pain, treated with indomethacin, colchicine, and his home dose of aspirin 81 mg daily. -

Electrophysiology with Artificial Intelligence Context and Challenge
Envisioning Cardiac Electrophysiology with Artificial Intelligence Context and Challenge An estimated 17 million people die of cardiovascular As technology advances, various industries are adopting diseases (CVDs) every year worldwide. CVD covers technologies such as digital transformation, internet of hypertension, sudden cardiac arrest, arrhythmia/rhythm things (IoT), artificial intelligence (AI), nanotechnology, disturbance, stroke, peripheral artery disease, and many and so on within their product/service portfolio and the more. Arrhythmias constitute a major problem, wherein the medical device industry is no exception. the heart beats either too quickly or too slowly or with an irregular pattern [1]. This indicates the malfunctioning of The focus of this paper is to discuss software-based the hearth’s electrical system. Clinical symptoms including solutions incorporating AI within EP systems that can shortness of breath, dizziness, sudden weakness, fluttering improve overall system performance, improve the in the chest, lightheadedness, and fainting, are indications therapeutic outcomes, reduce procedural time, and assist for malfunctioning of the heart. the electrophysiologist during the procedure. An electrophysiology (EP) study is a test to assess a person’s cardiac electrical activity. It helps the electrophysiologist to diagnose and determine the precise location and nature of arryhthmias. The test is performed by inserting catheters and wired electrodes to measure electrical activity through blood vessels that enter the heart. The two main goals of a cardiac EP study are (1) to accurately diagnosis the conduction-disturbance mechanism and (2) to determine the best line of treatment for the conduction-disturbances. Treatment following a cardiac EP study could range from ablation therapy to pharmacologic to device to surgical intervention based on the nature of the findings. -
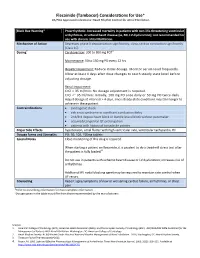
Flecainide Considerations For
Flecainide (Tambocor) Considerations for Use* US/FDA Approved Indications: Heart Rhythm Control for Atrial Fibrillation Black Box Warning* Proarrhythmic. Increased mortality in patients with non-life-threatening ventricular arrhythmias, structural heart disease (ie, MI, LV dysfunction); not recommended for use with chronic atrial fibrillation. Mechanism of Action Depresses phase 0 depolarization significantly, slows cardiac conduction significantly (Class 1C). Dosing† Cardioversion: 200 to 300 mg PO‡1 Maintenance: 50 to 150 mg PO every 12 hrs Hepatic Impairment: Reduce initial dosage. Monitor serum level frequently. Allow at least 4 days after dose changes to reach steady state level before adjusting dosage. Renal Impairment: CrCl > 35 ml/min: No dosage adjustment is required. CrCl <= 35 ml/min: Initially, 100 mg PO once daily or 50 mg PO twice daily. Adjust dosage at intervals > 4 days, since steady-state conditions may take longer to achieve in these patient Contraindications cardiogenic shock sick sinus syndrome or significant conduction delay 2nd/3rd degree heart block or bundle brand block without pacemaker acquired/congenital QT prolongation patients with history of torsade de pointes Major Side Effects hypotension, atrial flutter with high ventricular rate, ventricular tachycardia, HF Dosage forms and Strengths PO: 50, 100, 150mg tablets Special Notes Close monitoring of this drug is required. When starting a patient on flecainide, it is prudent to do a treadmill stress test after the patient is fully loaded.4 Do not use in patients with ischemic heart disease or LV dysfunction; increases risk of arrhythmias. Additional AV nodal blocking agent may be required to maintain rate control when AF recurs. -

About Electrophysiology Study of the Heart
About Electrophysiology Study of the Heart What is an Electrophysiology Study? An ElectroPhysiology (EP) Study is a test that looks at the electrical system of your heart. An EP Study will show if you have a heart rhythm problem and what is causing the problem. Heart rhythm problems are known as arrhythmias. Why is an EP Study done? An EP Study is done when you have problems such as fainting, dizziness, heart palpitations or an abnormal heart beat. How does the heart work? To understand this procedure, you need to know how the heart’s electrical system works. The sinoatrial node (SA node) is a natural pacemaker. It starts the electrical signal that travels across the upper 2 chambers or atria of the heart to the atrioventricular node (AV node). The AV node transfers the electrical signal from the upper part of the heart to the lower 2 pumping chambers or ventricles. The bundle branches are specialized tissues that help send electrical impulses through the ventricles. This makes a normal heart beat called normal sinus rhythm. 01-77-0623-0 (Rev 03/2016) Page 1 of 4 What causes heart rhythm problems? Problems happen when the heart beats too fast or too slow. Some people are born with heart rhythm problems. Problems may also be caused by aging or heart disease. There are many different kinds of arrhythmias. Problems occur when the heart beats too fast or too slow. When this happens you may feel: dizzy faint short of breath very tired palpitations (pounding in your chest) The treatment for heart rhythm problems may include one or more of the following: medication a pacemaker a defibrillator ablation Who will do the EP Study? A doctor who specializes in ElectroPhysiology (EP) will do the procedure. -

Discharge Advice After Atrial Fibrillation Ablation
Oxford University Hospitals NHS Trust Oxford Heart Centre Discharge advice after Atrial Fibrillation ablation Information for patients page 2 This booklet contains important advice about discharge after your Atrial Fibrillation (AF) ablation. It contains information about what to do when you get home. Contents 1. Discharge summary 4 Follow-up 4 Transport 4 2. What to do when you get home 5 Puncture site care 5 Bleeding 6 Sedation/General Anaesthetic 6 After the catheter ablation 6 Recurrence of AF symptoms - what to do 6 Driving 7 Return to work 8 3. Medication 8 4. How to contact us 9 5. Further information 10 6. Message for doctor reviewing this patient 10 page 3 1. Discharge summary Your Consultant at the John Radcliffe Hospital is: ………………………….…............................................................…….. Follow-up You will be sent an appointment for follow-up in the Arrhythmia clinic. (This appointment will be sent in the post. If you do not receive a date for an appointment within 8 weeks, please call the John Radcliffe Hospital and ask to speak to the secretary of your Consultant. Follow-up appointments are currently planned approximately 3 to 4 months after your procedure.) Transport to your outpatient appointments If you have difficulty getting to your outpatient appointments your GP surgery may have the phone numbers of voluntary transport schemes which operate at subsidised rates. A directory of these services is available at www.oxonrcc.org.uk for residents of the Oxfordshire area. page 4 2. What to do when you get home When you are discharged home, you should have a quiet few days resting to recover from your procedure. -

Resuscitation and Defibrillation
AARC GUIDELINE: RESUSCITATION AND DEFIBRILLATION AARC Clinical Practice Guideline Resuscitation and Defibrillation in the Health Care Setting— 2004 Revision & Update RAD 1.0 PROCEDURE: signs, level of consciousness, and blood gas val- Recognition of signs suggesting the possibility ues—included in those conditions are or the presence of cardiopulmonary arrest, initia- 4.1 Airway obstruction—partial or complete tion of resuscitation, and therapeutic use of de- 4.2 Acute myocardial infarction with cardio- fibrillation in adults. dynamic instability 4.3 Life-threatening dysrhythmias RAD 2.0 DESCRIPTION/DEFINITION: 4.4 Hypovolemic shock Resuscitation in the health care setting for the 4.5 Severe infections purpose of this guideline encompasses all care 4.6 Spinal cord or head injury necessary to deal with sudden and often life- 4.7 Drug overdose threatening events affecting the cardiopul- 4.8 Pulmonary edema monary system, and involves the identification, 4.9 Anaphylaxis assessment, and treatment of patients in danger 4.10 Pulmonary embolus of or in frank arrest, including the high-risk de- 4.11 Smoke inhalation livery patient. This includes (1) alerting the re- 4.12 Defibrillation is indicated when cardiac suscitation team and the managing physician; (2) arrest results in or is due to ventricular fibril- using adjunctive equipment and special tech- lation.1-5 niques for establishing, maintaining, and moni- 4.13 Pulseless ventricular tachycardia toring effective ventilation and circulation; (3) monitoring the electrocardiograph and recogniz- -

2018 EP Reimbursement and Coding Guide Physicians and Facilities Resources to Assist You with the Reimbursement Process!
2018 EP Reimbursement and Coding Guide Physicians and Facilities Resources to assist you with the Reimbursement Process! Reimbursement and Coding and Reimbursement Electrophysiology EP Procedure Documentation Coding Guide Frequently Asked Questions Coding Checklist Best Practices Online HCPCS C-Code Finder Coding & Reimbursement Webinars Email your Coding Questions www.biosensewebster.com/reimbursement Electrophysiology Diagnostic, Ablation, and Intracardiac Echocardiography Guided Transcatheter Procedures This guide has been developed to assist you in obtaining physician payment and hospital reimbursement for: • Electrophysiology (EP) diagnostic and ablation procedures • The acquisition of radiological images • EP and Cardiology procedures that may utilize intracardiac echocardiography (ICE) These procedures may be a covered service if they meet all of the requirements established by Medicare and private payers. It is essential that each claim be coded properly and supported with appropriate documentation in the medical record. TABLE OF CONTENTS PHYSICIAN SERVICES 4-6 CPT® Codes OUTPATIENT FACILITY SERVICES 7 Ambulatory Payment Classifications (APCs) INPATIENT FACILITY SERVICES 8 Medicare Severity Diagnosis Related Groups (MS-DRGs) PROCEDURE CODES 9-10 ICD-10-CM Procedure Codes DIAGNOSIS CODES 11-13 ICD-10-CM Diagnosis Codes HCPCS CODES FOR BIOSENSE WEBSTER, INC. PRODUCTS 14-15 NOTES 16 DISCLAIMER The information contained in this guide is provided to assist you in understanding the reimbursement process. It is intended to assist providers in accurately obtaining reimbursement for health care services. It is not intended to increase or maximize reimbursement by any payer. We strongly suggest that you consult your payer organization with regard to local reimbursement policies. The information contained in this document is provided for information purposes only and represents no statement, promise or guarantee by Biosense Webster, Inc. -

MANAGING ATRIAL FIBRILLATION (AF) (MODULE 2) MODULE 2: MANAGING ATRIAL FIBRILLATION Ii CONTENTS
YOUR COMPLETE GUIDE TO ATRIAL FIBRILLATION MANAGING ATRIAL FIBRILLATION (AF) (MODULE 2) MODULE 2: MANAGING ATRIAL FIBRILLATION ii CONTENTS iii Overview of managing AF 28 How are blood thinners used to reduce the risk of stroke in AF? 1 What are the goals of managing AF and atrial flutter? 31 What are the signs of a stroke? 2 Rate Control Strategy: 32 Being realistic when managing AF How is the heart rate controlled? (a) Medicine (b) Procedures 9 Rhythm Control Strategy: How is the heart rhythm controlled? (a) Medicine (b) Procedures 25 Reducing the risk of stroke: How is my stroke risk assessed? HEARTANDSTROKE.CA/AFGUIDE Published: February 2014 © 2014 Canadian Cardiovascular Society and Heart and Stroke Foundation of Canada. All rights reserved. Unauthorized use prohibited. MODULE 2: MANAGING ATRIAL FIBRILLATION iii OVERVIEW OF MANAGING AF AF Diagnosed MODULE 1 Find and Treat Common Causes What is it? Is it harmful? MODULE 2 Manage Arrhythmia Symptoms Assess Stroke Risk (CHADS2) Rate Control Rhythm Control • Medicine • Medicine Blood Thinner, Aspirin, or Nothing • Procedures • Procedures MODULE 3 Living Well with AF What to Expect Understanding Following a from Your AF Common Responses Patient Stories Healthy Lifestyle Management Plan and Finding Support FACT SHEET: OVERVIEW OF MANAGING AF HEARTANDSTROKE.CA/AFGUIDE Published: February 2014 © 2014 Canadian Cardiovascular Society and Heart and Stroke Foundation of Canada. All rights reserved. Unauthorized use prohibited. MODULE 2: MANAGING ATRIAL FIBRILLATION iv WHAT ARE TWO WAYS TO MANAGE AF? While AF is a chronic condition, it can be managed by: • medicine • procedures Medicine is usually tried first to manage symptoms caused by AF. -

Electrophysiology Study
Electrophysiology (EP) Study Highly trained specialists perform EP studies in a specially designed EP lab outfitted with advanced technology and equipment. Why an EP study? The Value of an EP Study While electrocardiograms (ECGs An electrophysiology, or EP, study or EKGs) are important tests of the provides information that is key to heart’s electrical system, they diagnosing and treating arrhythmias. provide only a brief snapshot of Although it is more invasive than an the heart’s electrical activity. electrocardiogram (ECG) or echocar - Arrhythmias can be unpredictable diogram, and involves provoking and intermittent, which makes it arrhythmias, the test produces data unlikely that an electrocardiogram that makes it possible to : will capture the underlying electri - Normally, electricity flows through - cal pathway problem. Even tests • Diagnose the source of arrhythmia out the heart in a regular, meas - that stretch over longer time periods , symptoms such as Holter monitoring, may not ured pattern. This electrical system • Evaluate the effectiveness of capture an event. brings about coordinated heart certain medications in controlling muscle contractions. A problem During an EP study, a specially the heart rhythm disorder anywhere along the electrical trained cardiac specialist may pro - • Predict the risk of a future cardiac pathway causes an arrhythmia, voke arrhythmia events and collect event, such as Sudden Cardiac or heart rhythm disturbance. By data about the flow of electricity Death accurately diagnosing the precise during actual events. As a result, cause of an arrhythmia, it is possi - • Assess the need for an implantable EP studies can diagnose the ble to select the best possible device (a pacemaker or ICD) or cause and precise location of the treatment. -

Stress, Protocols, and Tracers
ASNC IMAGING GUIDELINES ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers a b c Milena J. Henzlova, MD, W. Lane Duvall, MD, Andrew J. Einstein, MD, d e Mark I. Travin, MD, and Hein J. Verberne, MD a Mount Sinai Medical Center, New York, NY b Hartford Hospital, Hartford, CT c New York Presbyterian Hospital, Columbia University Medical Center, New York, NY d Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY e Academic Medical Center, Amsterdam, The Netherlands doi:10.1007/s12350-015-0387-x Abbreviations LEHR Low energy high resolution A2A Adenosine 2a LVEF Left ventricular ejection fraction AHA American Heart Association MBq Megabecquerels ALARA As low as reasonably achievable mCi Millicuries AV Atrioventricular MPI Myocardial perfusion imaging BP Blood pressure MRI Magnetic resonance imaging CBF Coronary blood flow mSv Millisievert CAD Coronary artery disease NE Norepinephrine CPET Cardiopulmonary exercise testing NET1 Norepinephrine transporter-1 DSP Deconvolution of septal penetration NPO Nil per os (nothing by mouth) ECG Electrocardiogram NYHA New York Heart Association EF Ejection fraction PET Positron emission tomography ESRD End-stage renal disease ROI Region of interest HF Heart failure SPECT Single-photon emission computed HFrEF Heart failure (with) reduced ejection tomography fraction TAVR Transcatheter aortic valve replacement HMR Heart-to-mediastinum ratio WR Washout rate ICD Implantable cardioversion defibrillator WPW Wolff-Parkinson White IV Intravenous