Spinal Facet Joint Biomechanics and Mechanotransduction in Normal, Injury and Degenerative
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Imaging Associates, Inc. Clinical Guidelines FACET JOINT
National Imaging Associates, Inc. Clinical guidelines Original Date: July 1, 2015 FACET JOINT INJECTIONS OR BLOCKS Page 1 of 4 “FOR FLORIDA BLUE MEMBERS ONLY” CPT Codes: Last Review Date: May 28, 2015 Cervical/Thoracic Region: 64490 (+ 64491, +64492) Lumbar/Sacral Region: 64493 (+64494, +64495) Medical Coverage Guideline Number: Last Revised Date: 02-61000-30 Responsible Department: Implementation Date: July 2015 Clinical Operations “FOR FLORIDA BLUE MEMBERS ONLY” INTRODUCTION Facet joints (also called zygapophysial joints or z-joints), are posterior to the vertebral bodies in the spinal column and connect the vertebral bodies to each other. They are located at the junction of the inferior articular process of a more cephalad vertebra, and the superior articular process of a more caudal vertebra. These joints provide stability and enable movement, allowing the spine to bend, twist, and extend in different directions. They also restrict hyperextension and hyperflexion. Facet joints are clinically important spinal pain generators in those with chronic spinal pain. Facet joints may refer pain to adjacent structures, making the underlying diagnosis difficult, as referred pain may assume a pseudoradicular pattern. Lumbar facet joints may refer pain to the back, buttocks, and lower extremities while cervical facet joints may refer pain to the head, neck and shoulders. Imaging findings are of little value in determining the source and location of ‘facet joint syndrome’, a term referring to back pain caused by pathology at the facet joints. Imaging studies may detect changes in facet joint architecture, but correlation between radiologic findings and symptoms is unreliable. Although clinical signs are also unsuitable for diagnosing facet joint-mediated pain, they may be of value in selecting candidates for controlled local anesthetic blocks of either the medial branches or the facet joint itself. -

The Erector Spinae Plane Block a Novel Analgesic Technique in Thoracic Neuropathic Pain
CHRONIC AND INTERVENTIONAL PAIN BRIEF TECHNICAL REPORT The Erector Spinae Plane Block A Novel Analgesic Technique in Thoracic Neuropathic Pain Mauricio Forero, MD, FIPP,*Sanjib D. Adhikary, MD,† Hector Lopez, MD,‡ Calvin Tsui, BMSc,§ and Ki Jinn Chin, MBBS (Hons), MMed, FRCPC|| Case 1 Abstract: Thoracic neuropathic pain is a debilitating condition that is often poorly responsive to oral and topical pharmacotherapy. The benefit A 67-year-old man, weight 116 kg and height 188 cm [body of interventional nerve block procedures is unclear due to a paucity of ev- mass index (BMI), 32.8 kg/m2] with a history of heavy smoking idence and the invasiveness of the described techniques. In this report, we and paroxysmal supraventricular tachycardia controlled on ateno- describe a novel interfascial plane block, the erector spinae plane (ESP) lol, was referred to the chronic pain clinic with a 4-month history block, and its successful application in 2 cases of severe neuropathic pain of severe left-sided chest pain. A magnetic resonance imaging (the first resulting from metastatic disease of the ribs, and the second from scan of his thorax at initial presentation had been reported as nor- malunion of multiple rib fractures). In both cases, the ESP block also pro- mal, and the working diagnosis at the time of referral was post- duced an extensive multidermatomal sensory block. Anatomical and radio- herpetic neuralgia. He reported constant burning and stabbing logical investigation in fresh cadavers indicates that its likely site of action neuropathic pain of 10/10 severity on the numerical rating score is at the dorsal and ventral rami of the thoracic spinal nerves. -

Pain Pattern Explanation Forms
Pain Pattern Explanation Forms 1. Cervical Facet Pain Pattern 2. Cervical Radicular/Dynatome Pain Pattern 3. Costotransverse Joint Pain Pattern 4. Fibromyalgia Points 5. Hip Joint Pain Pattern 6. Lumbar Dermatomes: Chemical Radiculitis 7. Lumbar Dermatomes: Disc Pathology 8. Lumbar Disc Pathology Healed 9. Lumbar Epidural Fibrosis 10. Lumbar Facet Pain Pattern 11. Lumbar Stenosis 12. Sacroiliac Joint Pain Pattern 13. Thoracic Facet Pain Pattern 14. Upper Cervical Joint Pain Pattern The OEA pain pattern handouts are PDF files that can be used for patient education and marketing. They help you explain your diagnosis with original illustrations that the patients can take home with them. There is limited text so you can tell your explanation of the treatment plan. The pain patterns can be printed in color or black and white. Once purchased, our business card will be replaced with yours to personalize each handout. Each illustration is based on the pain patterns that have been established in books or research articles when available. Normal anatomy and pathoanatomy illustrations are shown for the clinician to explain the diagnosis to the patient and how their treatment can influence the pain generator. These can also be utilized as marketing tools. The following pages are some guidelines that can be utilized to explain the handouts to patients. Cervical Facet Pain Pattern The cervical facet joints are the joints of the neck. Neurophysiologic studies have shown that cervical facet‐joint capsules are sources of neck pain.1 Dwyer et al.2 established pain patterns of the cervical facet joints. o Parasagittal cervical and cervicothoracic pain. -
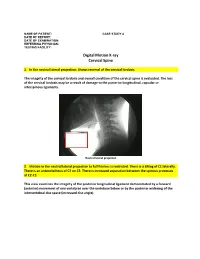
Digital Motion X-Ray Cervical Spine
NAME OF PATIENT: CASE STUDY 4 DATE OF REPORT: DATE OF EXAMINATION: REFERRING PHYSICIAN: TESTING FACILITY: Digital Motion X-ray Cervical Spine 1. In the neutral lateral projection: Shows reversal of the cervical lordosis. The integrity of the cervical lordosis and overall condition of the cervical spine is evaluated. The loss of the cervical lordosis may be a result of damage to the posterior longitudinal, capsular or interspinous ligaments. Neutral lateral projection 2. Motion in the neutral lateral projection to full flexion: Is restricted. There is a tilting of C1 laterally. There is an anterolisthesis of C2 on C3. There is increased separation between the spinous processes at C2-C3. This view examines the integrity of the posterior longitudinal ligament demonstrated by a forward (anterior) movement of one vertebrae over the vertebrae below or by the posterior widening of the intervertebral disc space (increased disc angle). Widening of posterior disc space Anterolisthesis The integrity of the interspinous ligament is evaluated in the lateral flexion view. Damage to this ligament results in increased separation of the spinous processes in flexion. Damaged Interspinous Ligament Full flexion projection 3. Motion in the neutral lateral projection to full extension: Is restricted. There is a retrolisthesis of C4 on C5. This view examines the integrity of the anterior longitudinal ligament demonstrated by a backward (posterior) movement of one vertebrae over the vertebrae below or by the anterior widening of the intervertebral disc space (increased disc angle). Retrolisthesis Widening of the anterior disc Full Extension 4. Motion in the oblique flexion projection: Is restricted. There is gapping of the facet joints at C6-C7 bilaterally and C7-T1 bilaterally. -

Relationship Between Endplate Defects, Modic Change, Facet Joint Degeneration, and Disc Degeneration of Cervical Spine
Neurospine 2020;17(2):443-452. Neurospine https://doi.org/10.14245/ns.2040076.038 pISSN 2586-6583 eISSN 2586-6591 Original Article Relationship Between Endplate Corresponding Author Defects, Modic Change, Facet Joint Dong Wuk Son https://orcid.org/0000-0002-9154-1923 Degeneration, and Disc Degeneration Department of Neurosurgery, Pusan of Cervical Spine National University Yangsan Hospital, 20 Geumo-ro, Mulgeum-eup, Yangsan 50612, Su-Hun Lee1,2, Dong Wuk Son1,2, Jun-Seok Lee1,2, Soon-Ki Sung1,2, Sang Weon Lee1,2, Korea Geun Sung Song1,2 E-mail: [email protected] 1Department of Neurosurgery, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea Received: February 11, 2020 2Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Revised: February 26, 2020 Hospital, Yangsan, Korea Accepted: February 27, 2020 Objective: The ‘‘disc degeneration precedes facet joint osteoarthritis’’ hypothesis and multi- dimensional analysis were actively discussed in lumbar spine. However, in cervical spine degeneration, the multifactorial analyzes of disc degeneration (DD), Modic changes (Mcs), facet degeneration, and endplate degeneration (ED) is still limited. In this cross-sectional study, we aimed to analyze the prevalence and interrelationship of cervical DD parameters. Methods: We retrospectively recruited 62 patients aged between 60 and 70 years. The disc height, segmental angle, ossified posterior longitudinal ligament (OPLL), ED, facet joint degeneration -

Ossification of the Posterior Longitudinal Ligament: Pathogenesis, Management, and Current Surgical Approaches
Neurosurg Focus 30 (3):E10, 2011 Ossification of the posterior longitudinal ligament: pathogenesis, management, and current surgical approaches A review ZACHARY A. SMITH, M.D.,1 COLIN C. BUCHANAN, M.D.,2 DAN RAPHAEL, P.A.-C.,1 AND LARRY T. KHOO, M.D.1 1Division of Neurosurgery, The Spine Clinic of Los Angeles, Good Samaritan Hospital, An Affiliate Hospital of the University of Southern California Medical School; and 2Department of Neurosurgery, Ronald Reagan–UCLA Medical Center, David Geffen School of Medicine at UCLA, Los Angeles, California Ossification of the posterior longitudinal ligament (OPLL) is an important cause of cervical myelopathy that results from bony ossification of the cervical or thoracic posterior longitudinal ligament (PLL). It has been estimated that nearly 25% of patients with cervical myelopathy will have features of OPLL. Patients commonly present in their mid-40s or 50s with clinical evidence of myelopathy. On MR and CT imaging, this can be seen as areas of ossification that commonly coalesce behind the cervical vertebral bodies, leading to direct ventral compression of the cord. While MR imaging will commonly demonstrate associated changes in the soft tissue, CT scanning will better define areas of ossification. This can also provide the clinician with evidence of possible dural ossification. The surgical management of OPLL remains a challenge to spine surgeons. Surgical alternatives include anterior, posterior, or circumferential decompression and/or stabilization. Anterior cervical stabilization options include cervical corpectomy or multilevel anterior cervical corpectomy and fusion, while posterior stabilization approaches include instrumented or noninstru- mented fusion or laminoplasty. Each of these approaches has distinct advantages and disadvantages. -

Ganglion Cyst of the Posterior Longitudinal Ligament Causing Lumbar Radiculopathy: Case Report
Spinal Cord (1997) 35, 632 ± 635 1997 International Medical Society of Paraplegia All rights reserved 1362 ± 4393/97 $12.00 Ganglion cyst of the posterior longitudinal ligament causing lumbar radiculopathy: case report Hisatoshi Baba1, Nobuaki Furusawa1, Yasuhisa Maezawa1, Kenzo Uchida1, Yasuo Kokubo1, Shinichi Imura1 and Sakon Noriki2 1Department of Orthopaedic Surgery; 2The First Department of Pathology, Fukui Medical School, Shimoaizuki 23, Matsuoka, Fukui 910-11, Japan We describe a man aged 26 years who presented with a neurological syndrome, which was found on lumbar radioculopathy to be due to a ganglion cyst originating from the posterior longitudinal ligament. Based on MRI ®ndings, a cystic lesion was suspected, a round lesion at L4 level with no connection to the adjacent facet or to the dura matter. During surgery, a liquid-containing cystic lesion was found to originate from the posterior longitudinal ligament at L4 level. The resected cyst was diagnosed histologically as a ganglion cyst. A complete cure was established after surgery and no recurrence was noted at a follow-up 1.7 years postoperatively. A ganglion cyst of the posterior longitudinal ligament should be considered in the dierential diagnosis of a cyst in the lumbar region causing neurological complications. Keywords: lumbar spine; posterior longitudinal ligament; ganglion cyst; radiculopathy Introduction Cystic lesions within the lumbosacral spinal canal can L4 dermatome. Muscle strength of the quadriceps cause symptoms and signs such as radiculopathy. femoris, tibialis anterior, extensor hallucis longus, was These cysts include ganglion1±3 and synovial cysts.4±7 assessed as normal. The straight leg raising test was To our knowledge, no report has previously been positive on the right at 508 and the femoral nerve described of a ganglion cyst in the posterior long- stretch test was also positive. -

Canine Thoracic Costovertebral and Costotransverse Joints Three Case Reports of Dysfunction and Manual Therapy Guidelines for A
Topics in Compan An Med 29 (2014) 1–5 Topical review Canine Thoracic Costovertebral and Costotransverse Joints: Three Case Reports of Dysfunction and Manual Therapy Guidelines for Assessment and Treatment of These Structures Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRTn Keywords: The costovertebral and costotransverse joints receive little attention in research. However, pain costovertebral associated with rib articulation dysfunction is reported to occur in human patients. The anatomic costotransverse structures of the canine rib joints and thoracic spine are similar to those of humans. As such, it is ribs physical therapy proposed that extrapolation from human physical therapy practice could be used for the assessment and rehabilitation treatment of the canine patient with presumed rib joint pain. This article presents 3 case studies that manual therapy demonstrate signs of rib dysfunction and successful treatment using primarily physical therapy manual techniques. General assessment and select treatment techniques are described. & 2014 Elsevier Inc. All rights reserved. The Canine Fitness Centre Ltd, Calgary, Alberta, Canada nAddress reprint requests to Laurie Edge-Hughes, BScPT, MAnimSt (Animal Physiotherapy), CAFCI, CCRT, The Canine Fitness Centre Ltd, 509—42nd Ave SE, Calgary, Alberta, Canada T2G 1Y7 E-mail: [email protected] The articular structures of the thorax comprise facet joints, the erect spine and further presented that in reviewing the literature, intervertebral disc, and costal joints. Little research has been they were unable to find mention of natural development of conducted on these joints in human or animal medicine. However, idiopathic scoliosis in quadrupeds; however, there are reports of clinical case presentations in human journals, manual therapy avian models and adolescent models in man. -

Posterior Longitudinal Ligament Status in Cervical Spine Bilateral Facet Dislocations
Thomas Jefferson University Jefferson Digital Commons Department of Orthopaedic Surgery Faculty Papers Department of Orthopaedic Surgery November 2005 Posterior longitudinal ligament status in cervical spine bilateral facet dislocations John A. Carrino Harvard Medical School & Brigham and Women's Hospital Geoffrey L. Manton Thomas Jefferson University Hospital William B. Morrison Thomas Jefferson University Hospital Alex R. Vaccaro Thomas Jefferson University Hospital and The Rothman Institute Mark E. Schweitzer New York University & Hospital for Joint Diseases Follow this and additional works at: https://jdc.jefferson.edu/orthofp Part of the Orthopedics Commons LetSee next us page know for additional how authors access to this document benefits ouy Recommended Citation Carrino, John A.; Manton, Geoffrey L.; Morrison, William B.; Vaccaro, Alex R.; Schweitzer, Mark E.; and Flanders, Adam E., "Posterior longitudinal ligament status in cervical spine bilateral facet dislocations" (2005). Department of Orthopaedic Surgery Faculty Papers. Paper 3. https://jdc.jefferson.edu/orthofp/3 This Article is brought to you for free and open access by the Jefferson Digital Commons. The Jefferson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The Commons is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The Jefferson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in Department of Orthopaedic Surgery Faculty Papers by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. -

Axially Loaded Magnetic Resonance Imaging Identification of the Factors
Journal of Clinical Medicine Article Axially Loaded Magnetic Resonance Imaging Identification of the Factors Associated with Low Back-Related Leg Pain Tomasz Lorenc 1 , Wojciech Michał Glinkowski 2,* and Marek Goł˛ebiowski 1 1 1st Department of Clinical Radiology, Medical University of Warsaw, 02-004 Warsaw, Poland; [email protected] (T.L.); [email protected] (M.G.) 2 Department of Medical Informatics and Telemedicine, Center of Excellence “TeleOrto” for Telediagnostics and Treatment of Disorders and Injuries of the Locomotor System, Medical University of Warsaw, 00-581 Warsaw, Poland * Correspondence: [email protected] Abstract: This retrospective observational study was conducted to identify factors associated with low back-related leg pain (LBLP) using axially loaded magnetic resonance imaging (AL-MRI). Ninety patients with low back pain (LBP) underwent AL-MRI of the lumbar spine. A visual analog scale and patient pain drawings were used to evaluate pain intensity and location and determine LBLP cases. The values of AL-MRI findings were analyzed using a logistic regression model with a binary dependent variable equal to one for low back-related leg pain and zero otherwise. Logistic regression results suggested that intervertebral joint effusion (odds ratio (OR) = 4.58; p = 0.035), atypical liga- menta flava (OR = 5.77; p = 0.003), and edema of the lumbar intervertebral joint (OR = 6.41; p = 0.003) p were more likely to be present in LBLP patients. Advanced disc degeneration ( = 0.009) and synovial cysts (p = 0.004) were less frequently observed in LBLP cases. According to the AL-MRI examinations, the odds of having LBLP are more likely if facet effusion, abnormal ligamenta flava, and lumbar Citation: Lorenc, T.; Glinkowski, W.M.; Goł˛ebiowski,M. -

Chapter 9 Vertebral Column Motion Segment Vertebral Joints Facet Joint Functions Intervertebral Discs
Vertebral Column • Curved stack of 33 vertebrate divided into Chapter 9 5 regions • Cerivcal Region – 7 Biomechanics of the Human Spine • Thoracic Region – 12 • Lumbar Region – 5 • Sacrum – 5 fused • Coccyx – 4 fused Motion Segment Vertebral Joints • 2 adjacent vertebrae and the associated soft tissues • Functional unit of the • Anterior spine – intervertebral symphysis joints • Posterior – Gliding diarthrodial facet joints Facet Joint Functions Intervertebral Discs • Channel and limit ROM in the different regions of • Fibrocartilaginous discs that cushion the anterior the spine spinal symphysis joints • Assist in lad bearing, sustaining up to 30% of the • Composed of a nucleus pulposus surrounded by compressive load on the spine the annulus fibrosus – Especially in hyperextension 1 Spinal Curves Spinal Movements • All three planes • circumduction • Lordosis – Exaggerated lumbar curve • Kyphosis – Exaggerated thoracic curve • Scoliosis – Lateral spinal curvature Cervical Flexors Abdominal Flexors • Rectus capitus anterior • Rectus abdominis • Rectus capitis lateralis • Internal obliques • Longus capitis • External obliques • Longus colli • 8 pairs of hyoid muscles Cervical Extension Thoracic/Lumbar Extensors • Erector Spinae • Splenius capitis – Iliocostalis – Longissimus – Spinalis • Splenius cervicis • Semispinalis – Capitis – Cervicis • Assisted by: – Thoracis – Rectus capitis • Deep Spinal Muscles posterior major/minor – Multifidi – Obliques capitis – Rotatores – Interspinales superior/inferior – Intertransversarii – Levatores cotarum -

The Role of Calcium Deposition in the Ligamentum Flavum Causing A
Paraplegia(1995) ll, 219-223 © 1995 International Medical Societyof Paraplegia All rightsreserved 0031-1758/95 $9.00 The role of calcium deposition in the ligamentum flavum causing a cauda equina syndrome and lumbar radiculopathy H Baba\ Y Maezawa1, N Furusawa1, S Imura1 and K Tomita2 IDepartment of Orthopaedic Surgery, Fukui Medical School, Shimoaizuki 23, Matsuoka, Fukui 910-11, Japan; 2Department of Orthopaedic Surgery, School of Medicine, Kanazawa University, Takaramachi 13-1, Kanazawa 920, Japan Our report concerns five patients who underwent lumbar decompressive surgery for cauda equina syndrome and radiculopathy secondary to degenerative stenosis associated with calcium deposition in the ligamentum flavum. The resected ligamentum flavum showed diffuse to massive calcium crystal deposition with a histologically marked degeneration in the elastic fibres. Energy dispensive radiographic microanalysis confirmed the crystal to be calcium pyrophosphate dihydrate in all patients, hydroxyapatite mixed in three and calcium orthophosphate mixed in one. The deposition appeared to be a change that is associated with the degenerative process in the ligament and, in some cases having marked deposition in a localised event, it causes or aggravates the neurological symptoms Keywords: lumbar spinal stenosis; cauda equina syndrome; radiculopathy; ligamentum flavum; calcium crystal deposition Introduction flavum with calcification compressed the dural sac at A number of reports in the literature describes the the levels suspected to be responsible for the cauda anatomical changes in the stenotic lumbar spine which equina syndrome and radiculopathy. According to produce the intermittent symptoms of cauda equina classification of Arnoldi et ai, 6 all patients showed a claudication and sciatica.1,2 The pathoanatomy in the degenerative type of stenosis radiologically; two had degenerative process includes hypertrophy of the mild spondylolisthesis anteriorly and one had scoliosis.