S-Cone Visual Stimuli Activate Superior Colliculus Neurons in Old World Monkeys: Implications for Understanding Blindsight
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neuro-Opthalmology (Developments in Ophthalmology, Vol
Neuro-Ophthalmology Developments in Ophthalmology Vol. 40 Series Editor W. Behrens-Baumann, Magdeburg Neuro- Ophthalmology Neuronal Control of Eye Movements Volume Editors Andreas Straube, Munich Ulrich Büttner, Munich 39 figures, and 3 tables, 2007 Basel · Freiburg · Paris · London · New York · Bangalore · Bangkok · Singapore · Tokyo · Sydney Andreas Straube Ulrich Büttner Department of Neurology Department of Neurology Klinikum Grosshadern Klinikum Grosshadern Marchioninistrasse 15 Marchioninistrasse 15 DE–81377 Munich DE–81377 Munich Library of Congress Cataloging-in-Publication Data Neuro-ophthalmology / volume editors, Andreas Straube, Ulrich Büttner. p. ; cm. – (Developments in ophthalmology, ISSN 0250-3751 ; v. 40) Includes bibliographical references and indexes. ISBN 978-3-8055-8251-3 (hardcover : alk. paper) 1. Neuroophthalmology. I. Straube, Andreas. II. Büttner, U. III. Series. [DNLM: 1. Eye Movements–physiology. 2. Ocular Motility Disorders. 3. Oculomotor Muscles–physiology. 4. Oculomotor Nerve-physiology. W1 DE998NG v.40 2007 / WW 400 N4946 2007] RE725.N45685 2007 617.7Ј32–dc22 2006039568 Bibliographic Indices. This publication is listed in bibliographic services, including Current Contents® and Index Medicus. Disclaimer. The statements, options and data contained in this publication are solely those of the individ- ual authors and contributors and not of the publisher and the editor(s). The appearance of advertisements in the book is not a warranty, endorsement, or approval of the products or services advertised or of their effectiveness, quality or safety. The publisher and the editor(s) disclaim responsibility for any injury to persons or property resulting from any ideas, methods, instructions or products referred to in the content or advertisements. Drug Dosage. The authors and the publisher have exerted every effort to ensure that drug selection and dosage set forth in this text are in accord with current recommendations and practice at the time of publication. -

Abstracts (PDF)
Abstracts of the Psychonomic Society — Volume 12 — November 2007 48th Annual Meeting — November 15–18, 2007 — Long Beach, California Papers 1–7 Friday Morning Implicit Memory is a widely used experimental procedure that has been modeled in Regency ABC, Friday Morning, 8:00–9:20 order to obtain ostensibly separate measures for explicit memory and implicit memory. Existing models are critiqued here and a new con- Chaired by Neil W. Mulligan fidence process-dissociation (CPD) model is developed for a modi- University of North Carolina, Chapel Hill fied process-dissociation task that uses confidence ratings in con- junction with the usual old/new recognition response. With the new 8:00–8:15 (1) model there are several direct tests for the key assumptions of the stan- Attention and Auditory Implicit Memory. NEIL W. MULLIGAN, dard models for this task. Experimental results are reported here that University of North Carolina, Chapel Hill—Traditional theorizing are contrary to the critical assumptions of the earlier models for the stresses the importance of attentional state during encoding for later process-dissociation task. These results cast doubt on the suitability memory, based primarily on research with explicit memory. Recent re- of the process-dissociation procedure itself for obtaining separate search has investigated the role of attention in implicit memory in the measures for implicit and explicit memory. visual modality. The present experiments examined the effect of divided attention on auditory implicit memory, using auditory perceptual iden- Attentional Selection and Priming tification, word-stem completion, and word-fragment completion. Par- Regency DEFH, Friday Morning, 8:00–10:00 ticipants heard study words under full attention conditions or while si- multaneously carrying out a distractor task. -

Cognition Revised Edition 2015
IIID Public Library Information Design 5 Cognition Revised edition 2015 Rune Pettersson Institute for infology IIID Public Library The “IIID Public Library” is a free resource for all who are interested in information design. This book was kindly donated by the author free of charge to visitors of the IIID Public Library / Website. International Institute for Information Design (IIID) designforum Wien, MQ/quartier 21 Museumsplatz 1, 1070 Wien, Austria www.iiid.net Information Design 5 Cognition Words Pictures Rune Pettersson * Institute for infology Information Design 5–Cognition Yin and yang, or yin-yang, is a concept used in Chinese philoso- phy to describe how seemingly opposite forces are intercon- nected and interdependent, and how they give rise to each other. Many natural dualities, such as life and death, light and dark, are thought of as physical manifestations of the concept. Yin and yang can also be thought of as complementary forces interacting to form a dynamic system in which the whole is greater than the parts. In information design, theory and prac- tice is an example where the whole is greater than the parts. In this book drawings and photos are my own, unless other information. ISBN 978-91-85334-30-8 © Rune Pettersson Tullinge 2015 2 Preface Information design is a multi-disciplinary, multi-dimensional, and worldwide consideration with influences from areas such as language, art and aesthetics, information, communication, be- haviour and cognition, business and law, as well as media pro- duction technologies. Since my retirement I have edited and revised sections of my earlier books, conference papers and reports about informa- tion design, message design, visual communication and visual literacy. -

Getting Signals Into the Brain: Visual Prosthetics Through Thalamic Microstimulation
Neurosurg Focus 27 (1):E6, 2009 Getting signals into the brain: visual prosthetics through thalamic microstimulation JOHN S. PEZARI S , PH.D., AN D EMA D N. ES KAN D AR , M.D. Department of Neurosurgery, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts Common causes of blindness are diseases that affect the ocular structures, such as glaucoma, retinitis pigmen- tosa, and macular degeneration, rendering the eyes no longer sensitive to light. The visual pathway, however, as a pre- dominantly central structure, is largely spared in these cases. It is thus widely thought that a device-based prosthetic approach to restoration of visual function will be effective and will enjoy similar success as cochlear implants have for restoration of auditory function. In this article the authors review the potential locations for stimulation electrode placement for visual prostheses, assessing the anatomical and functional advantages and disadvantages of each. Of particular interest to the neurosurgical community is placement of deep brain stimulating electrodes in thalamic structures that has shown substantial promise in an animal model. The theory of operation of visual prostheses is discussed, along with a review of the current state of knowledge. Finally, the visual prosthesis is proposed as a model for a general high-fidelity machine-brain interface.(DOI: 10.3171/2009.4.FOCUS0986) KEY WOR ds • visual prosthesis • deep brain stimulation • visual function N this article we review the current state of visual Evaluating Points Along the Early prosthetics with particular attention to the approaches Visual Pathway as Stimulation Targets that include neurosurgical methodologies. I There are 6 locations along the visual pathway with potential for functional restoration of sight through mi- Background: Causes of Blindness crostimulation as depicted in Fig. -
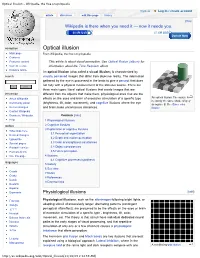
Optical Illusion - Wikipedia, the Free Encyclopedia
Optical illusion - Wikipedia, the free encyclopedia Try Beta Log in / create account article discussion edit this page history [Hide] Wikipedia is there when you need it — now it needs you. $0.6M USD $7.5M USD Donate Now navigation Optical illusion Main page From Wikipedia, the free encyclopedia Contents Featured content This article is about visual perception. See Optical Illusion (album) for Current events information about the Time Requiem album. Random article An optical illusion (also called a visual illusion) is characterized by search visually perceived images that differ from objective reality. The information gathered by the eye is processed in the brain to give a percept that does not tally with a physical measurement of the stimulus source. There are three main types: literal optical illusions that create images that are interaction different from the objects that make them, physiological ones that are the An optical illusion. The square A About Wikipedia effects on the eyes and brain of excessive stimulation of a specific type is exactly the same shade of grey Community portal (brightness, tilt, color, movement), and cognitive illusions where the eye as square B. See Same color Recent changes and brain make unconscious inferences. illusion Contact Wikipedia Donate to Wikipedia Contents [hide] Help 1 Physiological illusions toolbox 2 Cognitive illusions 3 Explanation of cognitive illusions What links here 3.1 Perceptual organization Related changes 3.2 Depth and motion perception Upload file Special pages 3.3 Color and brightness -

Short Wavelength Cone Activity to and Through the Superior Colliculus
SHORT WAVELENGTH CONE ACTIVITY TO AND THROUGH THE SUPERIOR COLLICULUS by Nathan Hall B.S., University of Pittsburgh, 2007 Submitted to the Graduate Faculty of The Kenneth P. Dietrich School of Arts and Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Pittsburgh 2016 UNIVERSITY OF PITTSBURGH KENNETH P. DIETRICH SCHOOL OF ARTS AND SCIENCES This dissertation was presented by Nathan Hall It was defended on March 22, 2016 and approved by Daniel J. Simons, PhD, Department of Neurobiology Marc A. Sommer, PhD, Department of Biomedical Engineering, Duke University Marlene R. Cohen, PhD, Department of Neuroscience Matthew A. Smith, PhD, Department of Ophthalmology Neeraj J. Gandhi, PhD, Department of Bioengineering Dissertation Advisor: Carol L. Colby, PhD, Department of Neuroscience ii Copyright © by Nathan Hall 2016 iii SHORT WAVELENGTH CONE ACTIVITY TO AND THROUGH THE SUPERIOR COLLICULUS Nathan Hall, PhD University of Pittsburgh, 2016 A key structure for directing saccadic eye movements is the superior colliculus (SC). The SC is thought to be unresponsive to stimuli that activate only short wavelength sensitive cones (S-cones) in the retina. The apparent lack of S-cone input to the SC was recognized as an opportunity to test SC function. The assumption that S-cone stimuli are invisible to the SC has been used in numerous human clinical and psychophysical studies. The idea is that visually guided behavior dependent on the SC should be impaired when S-cone stimuli are used. Behavioral impairment to S-cone stimuli is used to infer the role of the SC in a given behavior. -

A Dictionary of Neurological Signs.Pdf
A DICTIONARY OF NEUROLOGICAL SIGNS THIRD EDITION A DICTIONARY OF NEUROLOGICAL SIGNS THIRD EDITION A.J. LARNER MA, MD, MRCP (UK), DHMSA Consultant Neurologist Walton Centre for Neurology and Neurosurgery, Liverpool Honorary Lecturer in Neuroscience, University of Liverpool Society of Apothecaries’ Honorary Lecturer in the History of Medicine, University of Liverpool Liverpool, U.K. 123 Andrew J. Larner MA MD MRCP (UK) DHMSA Walton Centre for Neurology & Neurosurgery Lower Lane L9 7LJ Liverpool, UK ISBN 978-1-4419-7094-7 e-ISBN 978-1-4419-7095-4 DOI 10.1007/978-1-4419-7095-4 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2010937226 © Springer Science+Business Media, LLC 2001, 2006, 2011 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omissions that may be made. -

Tractographic Methods Employed in the Visual Pathway
CHARACTERISATION OF THE OPTIC RADIATIONS IN CHILDREN IN HEALTH AND DISEASE Say Ayala Soriano A thesis submitted for the degree of Doctor of Philosophy University College London January 2016 1 DECLARATION I, Say Ayala Soriano, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. 2 ABSTRACT The normal and abnormal development of the optic radiations through childhood was examined in terms of their anatomical development, using MRI tractography, and their functional development, using visual evoked potentials (VEPs). Neurosurgical applications of these imaging techniques were assessed. Control cohorts of 74 children and 13 adults were recruited from Great Ormond Street Hospital. The anatomical development of the optic radiations in children from birth was described using tractography. A novel method to improve tractography analysis using VEP data was developed. VEP-enhanced tractography showed a more defined optic radiation in the gathering of the visual cortex, which caused a significant reduction in the mean FA in the adult cohort. Paediatric patients diagnosed with optic nerve hypoplasia (ONH) were recruited and 23 were compared with a matched control cohort using tractography. ONH patients presented reduced mean FA in the left optic radiation. TBSS analysis of the DTI scans showed that white matter FA was also lower in other areas of the brain outside of the visual system. Two paediatric seizure patient cohorts were recruited: 21 patients with a single episode of prolonged febrile convulsions and 20 regular users of anti-epileptic medicines. Both cohorts were compared with matched control cohorts using DTI tractography. -

Disorders of Pupillary Function, Accommodation, and Lacrimation
CHAPTER 16 Disorders of Pupillary Function, Accommodation, and Lacrimation Aki Kawasaki DISORDERS OF THE PUPIL DISORDERS OF LACRIMATION Structural Defects of the Iris Hypolacrimation Afferent Abnormalities Hyperlacrimation Efferent Abnormalities: Anisocoria Inappropriate Lacrimation Disturbances in Disorders of the Neuromuscular Junction Drug Effects on Lacrimation Drug Effects GENERALIZED DISTURBANCES OF AUTONOMIC FUNCTION Light–Near Dissociation Ross Syndrome Disturbances During Seizures Familial Dysautonomia Disturbances During Coma Shy-Drager Syndrome DISORDERS OF ACCOMMODATION Autoimmune Autonomic Neuropathy Accommodation Insufficiency and Paralysis Miller Fisher Syndrome Accommodation Spasm and Spasm of the Near Reflex Drug Effects on Accommodation In this chapter I describe various disorders that produce mation. Although many of these disorders are isolated dysfunction of the autonomic nervous system as it pertains phenomena that affect only a single structure, others are to the eye and orbit, including congenital and acquired systemic disorders that involve various other organs in the disorders of pupillary function, accommodation, and lacri- body. DISORDERS OF THE PUPIL The value of observation of pupillary size and motility in and reactivity because these structural defects may be the the evaluation of patients with neurologic disease cannot cause of ‘‘abnormal pupils’’ and often are easy to diagnose be overemphasized. In many patients with visual loss, an at the slit lamp. Furthermore, if a preexisting structural iris abnormal pupillary response is the only objective sign of defect is present, it may confound interpretation of the neuro- organic visual dysfunction. In patients with diplopia, an im- logic evaluation of pupillary function; at the very least, it paired pupil can signal the presence of an acute or enlarging should be kept in consideration during such evaluation. -
The Sander Parallelogram Illusion Dissociates Action and Perception Despite Control for the Litany of Past Confounds
cortex 98 (2018) 163e176 Available online at www.sciencedirect.com ScienceDirect Journal homepage: www.elsevier.com/locate/cortex Special issue: Research report The Sander parallelogram illusion dissociates action and perception despite control for the litany of past confounds * Robert L. Whitwell a, , Melvyn A. Goodale b,c, Kate E. Merritt b and James T. Enns a a Department of Psychology, The University of British Columbia, Canada b The Brain and Mind Institute, The University of Western Ontario, Canada c Department of Psychology, The University of Western Ontario, Canada article info abstract Article history: The two visual systems hypothesis proposes that human vision is supported by an Received 3 October 2016 occipito-temporal network for the conscious visual perception of the world and a fronto- Reviewed 09 November 2016 parietal network for visually-guided, object-directed actions. Two specific claims about Revised 7 February 2017 the fronto-parietal network's role in sensorimotor control have generated much data and Accepted 20 September 2017 controversy: (1) the network relies primarily on the absolute metrics of target objects, Published online 5 October 2017 which it rapidly transforms into effector-specific frames of reference to guide the fingers, hands, and limbs, and (2) the network is largely unaffected by scene-based information Keywords: extracted by the occipito-temporal network for those same targets. These two claims lead Visual illusion to the counter-intuitive prediction that in-flight anticipatory configuration of the fingers Sander Parallelogram during object-directed grasping will resist the influence of pictorial illusions. The research Action confirming this prediction has been criticized for confounding the difference between Perception grasping and explicit estimates of object size with differences in attention, sensory feed- Grasping back, obstacle avoidance, metric sensitivity, and priming. -

From Gestalt Theory to Image Analysis
From Gestalt Theory to Image Analysis A Probabilistic Approach Agn`es Desolneux Lionel Moisan Jean-Michel Morel October 2007 Preface The theory in these notes was taught between 2002 and 2005 at the graduate schools of Ecole Nor- male Sup´erieure de Cachan, Ecole Polytechnique de Palaiseau, Universitat Pompeu Fabra, Barcelona, Universitat de les Illes of Balears, Palma, and University of California at Los Angeles. It is also being taught by Andr`es Almansa at the Facultad de Ingeneria, Montevideo. This text will be of interest to several kinds of audience. Our teaching experience proves that specialists in image analysis and computer vision find the text easy at the computer vision side and accessible on the mathematical level. The prerequisites are elementary calculus and probability from the first two undergraduate years of any science course. All slightly more advanced notions in probability (inequalities, stochastic geometry, large deviations, etc.) will be either proved in the text or detailed in several exercises at the end of each chapter. We have always asked the students to do all exercises and they usually succeed regardless of what their science background is. The mathematics students do not find the mathematics difficult and easily learn through the text itself what is needed in vision psychology and the practice of computer vision. The text aims at being self-contained in all three aspects: mathematics, vision, and algorithms. We will in particular explain what a digital image is and how the elementary structures can be computed. We wish to emphasize why we are publishing these notes in a mathematics collection. -
2012 Program Meeting Schedule
Vision Sciences Society 12th Annual Meeting, May 11-16, 2012 Waldorf Astoria Naples, Naples, Florida Program Contents Board, Review Committee & Staff . 2 Saturday Morning Talks . 33 Featured Events . 3 Saturday Monring Posters. 34 VSS @ ARVO Symposium . 3 Saturday Afternoon Talks . 38 Meeting Schedule . 4 Saturday Afternoon Posters . 39 Schedule-at-a-Glance . 6 Sunday Morning Talks. 43 Poster Schedule . 8 Sunday Morning Posters . 44 Talk Schedule . 10 Sunday Afternoon Talks . 48 Keynote Address . 11 Sunday Afternoon Posters. 49 Elsevier/VSS Young Investigator Award. 12 Monday Morning Talks . 53 Funding Workshops . 13 Monday Morning Posters . 54 Club Vision Dance Party. 13 Tuesday Morning Talks . 58 Satellite Events. 14 Tuesday Morning Posters . 59 Elsevier/Vision Research Travel Awards 15 Tuesday Afternoon Talks . 63 Student Events . 16 Tuesday Afternoon Posters . 64 VSS Public Lecture . 17 Wednesday Morning Talks . 68 Attendee Resources . 18 Wednesday Morning Posters . 69 Exhibitors . 21 Topic Index. 72 VSS Dinner and Demo Night . 24 Author Index . 75 Member-Initiated Symposia . 27 Hotel Floorplan . 91 Friday Evening Posters . 30 Advertisements. 93 Program and Abstracts cover design by Asieh Zadbood, Brain and Cognitive Science Dept., Seoul National University, Psychology Dept., Vanderbilt University T-shirt design and page graphics by Jeffrey Mulligan, NASA Ames Research Center Board, Review Committee & Staff Board of Directors Marisa Carrasco (2013), President Mary Hayhoe (2015) New York University University of Texas, Austin Karl