Drawing Inspiration from Biological Dendrites to Empower Artificial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
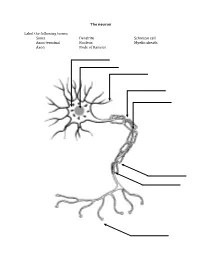
The Neuron Label the Following Terms: Soma Axon Terminal Axon Dendrite
The neuron Label the following terms: Soma Dendrite Schwaan cell Axon terminal Nucleus Myelin sheath Axon Node of Ranvier Neuron Vocabulary You must know the definitions of these terms 1. Synaptic Cleft 2. Neuron 3. Impulse 4. Sensory Neuron 5. Motor Neuron 6. Interneuron 7. Body (Soma) 8. Dendrite 9. Axon 10. Action Potential 11. Myelin Sheath (Myelin) 12. Afferent Neuron 13. Threshold 14. Neurotransmitter 15. Efferent Neurons 16. Axon Terminal 17. Stimulus 18. Refractory Period 19. Schwann 20. Nodes of Ranvier 21. Acetylcholine STEPS IN THE ACTION POTENTIAL 1. The presynaptic neuron sends neurotransmitters to postsynaptic neuron. 2. Neurotransmitters bind to receptors on the postsynaptic cell. - This action will either excite or inhibit the postsynaptic cell. - The soma becomes more positive. 3. The positive charge reaches the axon hillock. - Once the threshold of excitation is reached the neuron will fire an action potential. 4. Na+ channels open and Na+ is forced into the cell by the concentration gradient and the electrical gradient. - The neuron begins to depolarize. 5. The K+ channels open and K+ is forced out of the cell by the concentration gradient and the electrical gradient. - The neuron is depolarized. 6. The Na+ channels close at the peak of the action potential. - The neuron starts to repolarize. 7. The K+ channels close, but they close slowly and K+ leaks out. 8. The terminal buttons release neurotransmitter to the postsynaptic neuron. 9. The resting potential is overshot and the neuron falls to a -90mV (hyperpolarizes). - The neuron continues to repolarize. 10. The neuron returns to resting potential. The Synapse Label the following terms: Pre-synaptic membrane Neurotransmitters Post-synaptic membrane Synaptic cleft Vesicle Post-synaptic receptors . -

The Synaptic Weight Matrix
The Synaptic Weight Matrix: Dynamics, Symmetry Breaking, and Disorder Francesco Fumarola Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2018 © 2018 Francesco Fumarola All rights reserved ABSTRACT The Synaptic Weight Matrix: Dynamics, Symmetry Breaking, and Disorder Francesco Fumarola A key role in simplified models of neural circuitry (Wilson and Cowan, 1972) is played by the matrix of synaptic weights, also called connectivity matrix, whose elements describe the amount of influence the firing of one neuron has on another. Biologically, this matrix evolves over time whether or not sensory inputs are present, and symmetries possessed by the internal dynamics of the network may break up sponta- neously, as found in the development of the visual cortex (Hubel and Wiesel, 1977). In this thesis, a full analytical treatment is provided for the simplest case of such a biologi- cal phenomenon, a single dendritic arbor driven by correlation-based dynamics (Linsker, 1988). Borrowing methods from the theory of Schrödinger operators, a complete study of the model is performed, analytically describing the break-up of rotational symmetry that leads to the functional specialization of cells. The structure of the eigenfunctions is calcu- lated, lower bounds are derived on the critical value of the correlation length, and explicit expressions are obtained for the long-term profile of receptive fields, i.e. the dependence of neural activity on external inputs. The emergence of a functional architecture of orientation preferences in the cortex is another crucial feature of visual information processing. -

Crayfish Escape Behavior and Central Synapses
Crayfish Escape Behavior and Central Synapses. III. Electrical Junctions and Dendrite Spikes in Fast Flexor Motoneurons ROBERT S. ZUCKER Department of Biological Sciences and Program in the Neurological Sciences, Stanford University, Stanford, California 94305 THE LATERAL giant fiber is the decision fiber and fast flexor motoneurons are located in for a behavioral response in crayfish in- the dorsal neuropil of each abdominal gan- volving rapid tail flexion in response to glion. Dye injections and anatomical recon- abdominal tactile stimulation (27). Each structions of ‘identified motoneurons reveal lateral giant impulse is followed by a rapid that only those motoneurons which have tail flexion or tail flip, and when the giant dentritic branches in contact with a giant fiber does not fire in response to such stim- fiber are activated by that giant fiber (12, uli, the movement does not occur. 21). All of tl ie fast flexor motoneurons are The afferent limb of the reflex exciting excited by the ipsilateral giant; all but F4, the lateral giant has been described (28), F5 and F7 are also excited by the contra- and the habituation of the behavior has latkral giant (21 a). been explained in terms of the properties The excitation of these motoneurons, of this part of the neural circuit (29). seen as depolarizing potentials in their The properties of the neuromuscular somata, does not always reach threshold for junctions between the fast flexor motoneu- generating a spike which propagates out the rons and the phasic flexor muscles have also axon to the periphery (14). Indeed, only t,he been described (13). -

Plp-Positive Progenitor Cells Give Rise to Bergmann Glia in the Cerebellum
Citation: Cell Death and Disease (2013) 4, e546; doi:10.1038/cddis.2013.74 OPEN & 2013 Macmillan Publishers Limited All rights reserved 2041-4889/13 www.nature.com/cddis Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum S-H Chung1, F Guo2, P Jiang1, DE Pleasure2,3 and W Deng*,1,3,4 NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre–Lox systems in vivo with two different mouse lines, the Plp-Cre-ERT2/Rosa26-EYFP and Olig2-Cre-ERT2/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2 þ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2 þ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic–ischemic injury, but the ability of NG2 þ cells to give rise to Bergmann glia and astrocytes remains unchanged. -

Neuregulin 1–Erbb2 Signaling Is Required for the Establishment of Radial Glia and Their Transformation Into Astrocytes in Cerebral Cortex
Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex Ralf S. Schmid*, Barbara McGrath*, Bridget E. Berechid†, Becky Boyles*, Mark Marchionni‡, Nenad Sˇ estan†, and Eva S. Anton*§ *University of North Carolina Neuroscience Center and Department of Cell and Molecular Physiology, University of North Carolina School of Medicine, Chapel Hill, NC 27599; †Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06510; and ‡CeNes Pharamceuticals, Inc., Norwood, MA 02062 Communicated by Pasko Rakic, Yale University School of Medicine, New Haven, CT, January 27, 2003 (received for review December 12, 2002) Radial glial cells and astrocytes function to support the construction mine whether NRG-1-mediated signaling is involved in radial and maintenance, respectively, of the cerebral cortex. However, the glial cell development and differentiation in the cerebral cortex. mechanisms that determine how radial glial cells are established, We show that NRG-1 signaling, involving erbB2, may act in maintained, and transformed into astrocytes in the cerebral cortex are concert with Notch signaling to exert a critical influence in the not well understood. Here, we show that neuregulin-1 (NRG-1) exerts establishment, maintenance, and appropriate transformation of a critical role in the establishment of radial glial cells. Radial glial cell radial glial cells in cerebral cortex. generation is significantly impaired in NRG mutants, and this defect can be rescued by exogenous NRG-1. Down-regulation of expression Materials and Methods and activity of erbB2, a member of the NRG-1 receptor complex, leads Clonal Analysis to Study NRG’s Role in the Initial Establishment of to the transformation of radial glial cells into astrocytes. -

Behavioral Plasticity Through the Modulation of Switch Neurons
Behavioral Plasticity Through the Modulation of Switch Neurons Vassilis Vassiliades, Chris Christodoulou Department of Computer Science, University of Cyprus, 1678 Nicosia, Cyprus Abstract A central question in artificial intelligence is how to design agents ca- pable of switching between different behaviors in response to environmental changes. Taking inspiration from neuroscience, we address this problem by utilizing artificial neural networks (NNs) as agent controllers, and mecha- nisms such as neuromodulation and synaptic gating. The novel aspect of this work is the introduction of a type of artificial neuron we call \switch neuron". A switch neuron regulates the flow of information in NNs by se- lectively gating all but one of its incoming synaptic connections, effectively allowing only one signal to propagate forward. The allowed connection is determined by the switch neuron's level of modulatory activation which is affected by modulatory signals, such as signals that encode some informa- tion about the reward received by the agent. An important aspect of the switch neuron is that it can be used in appropriate \switch modules" in or- der to modulate other switch neurons. As we show, the introduction of the switch modules enables the creation of sequences of gating events. This is achieved through the design of a modulatory pathway capable of exploring in a principled manner all permutations of the connections arriving on the switch neurons. We test the model by presenting appropriate architectures in nonstationary binary association problems and T-maze tasks. The results show that for all tasks, the switch neuron architectures generate optimal adaptive behaviors, providing evidence that the switch neuron model could be a valuable tool in simulations where behavioral plasticity is required. -

Neuron Morphology Influences Axon Initial Segment Plasticity
Dartmouth College Dartmouth Digital Commons Dartmouth Scholarship Faculty Work 1-2016 Neuron Morphology Influences Axon Initial Segment Plasticity Allan T. Gulledge Dartmouth College, [email protected] Jaime J. Bravo Dartmouth College Follow this and additional works at: https://digitalcommons.dartmouth.edu/facoa Part of the Computational Neuroscience Commons, Molecular and Cellular Neuroscience Commons, and the Other Neuroscience and Neurobiology Commons Dartmouth Digital Commons Citation Gulledge, A.T. and Bravo, J.J. (2016) Neuron morphology influences axon initial segment plasticity, eNeuro doi:10.1523/ENEURO.0085-15.2016. This Article is brought to you for free and open access by the Faculty Work at Dartmouth Digital Commons. It has been accepted for inclusion in Dartmouth Scholarship by an authorized administrator of Dartmouth Digital Commons. For more information, please contact [email protected]. New Research Neuronal Excitability Neuron Morphology Influences Axon Initial Segment Plasticity1,2,3 Allan T. Gulledge1 and Jaime J. Bravo2 DOI:http://dx.doi.org/10.1523/ENEURO.0085-15.2016 1Department of Physiology and Neurobiology, Geisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire 03756, and 2Thayer School of Engineering at Dartmouth, Hanover, New Hampshire 03755 Visual Abstract In most vertebrate neurons, action potentials are initiated in the axon initial segment (AIS), a specialized region of the axon containing a high density of voltage-gated sodium and potassium channels. It has recently been proposed that neurons use plasticity of AIS length and/or location to regulate their intrinsic excitability. Here we quantify the impact of neuron morphology on AIS plasticity using computational models of simplified and realistic somatodendritic morphologies. -

Satellite Glial Cell Communication in Dorsal Root Ganglia
Neuronal somatic ATP release triggers neuron– satellite glial cell communication in dorsal root ganglia X. Zhang, Y. Chen, C. Wang, and L.-Y. M. Huang* Department of Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX 77555-1069 Edited by Charles F. Stevens, The Salk Institute for Biological Studies, La Jolla, CA, and approved April 25, 2007 (received for review December 14, 2006) It has been generally assumed that the cell body (soma) of a release of ATP from the somata of DRG neurons. Here, we show neuron, which contains the nucleus, is mainly responsible for that electric stimulation elicits robust vesicular release of ATP from synthesis of macromolecules and has a limited role in cell-to-cell neuronal somata and thus triggers bidirectional communication communication. Using sniffer patch recordings, we show here that between neurons and satellite cells. electrical stimulation of dorsal root ganglion (DRG) neurons elicits robust vesicular ATP release from their somata. The rate of release Results events increases with the frequency of nerve stimulation; external We asked first whether vesicular release of ATP occurs in the -Ca2؉ entry is required for the release. FM1–43 photoconversion somata of DRG neurons. P2X2-EGFP receptors were overex analysis further reveals that small clear vesicles participate in pressed in HEK cells and used as the biosensor (i.e., sniffer patch exocytosis. In addition, the released ATP activates P2X7 receptors method) (20). P2X2 receptors were chosen because they were easily in satellite cells that enwrap each DRG neuron and triggers the expressed at a high level (Ϸ200 pA/pF) in HEK cells and the communication between neuronal somata and glial cells. -

Was Not Reached, However, Even After Six to Sevenhours. A
PROTEIN SYNTHESIS IN THE ISOLATED GIANT AXON OF THE SQUID* BY A. GIUDITTA,t W.-D. DETTBARN,t AND MIROSLAv BRZIN§ MARINE BIOLOGICAL LABORATORY, WOODS HOLE, MASSACHUSETTS Communicated by David Nachmansohn, February 2, 1968 The work of Weiss and his associates,1-3 and more recently of a number of other investigators,4- has established the occurrence of a flux of materials from the soma of neurons toward the peripheral regions of the axon. It has been postulated that this mechanism would account for the origin of most of the axonal protein, although the time required to cover the distance which separates some axonal tips from their cell bodies would impose severe delays.4 On the other hand, a number of observations7-9 have indicated the occurrence of local mechanisms of synthesis in peripheral axons, as suggested by the kinetics of appearance of individual proteins after axonal transection. In this paper we report the incorporation of radioactive amino acids into the protein fraction of the axoplasm and of the axonal envelope obtained from giant axons of the squid. These axons are isolated essentially free from small fibers and connective tissue, and pure samples of axoplasm may be obtained by extru- sion of the axon. Incorporation of amino acids into axonal protein has recently been reported using systems from mammals'0 and fish."I Materials and Methods.-Giant axons of Loligo pealii were dissected and freed from small fibers: they were tied at both ends. Incubations were carried out at 18-20° in sea water previously filtered through Millipore which contained 5 mM Tris pH 7.8 and 10 Muc/ml of a mixture of 15 C'4-labeled amino acids (New England Nuclear Co., Boston, Mass.). -

The Question of Algorithmic Personhood and Being
Article The Question of Algorithmic Personhood and Being (Or: On the Tenuous Nature of Human Status and Humanity Tests in Virtual Spaces—Why All Souls Are ‘Necessarily’ Equal When Considered as Energy) Tyler Lance Jaynes Alden March Bioethics Institute, Albany Medical College, Albany, NY 12208, USA; [email protected] Abstract: What separates the unique nature of human consciousness and that of an entity that can only perceive the world via strict logic-based structures? Rather than assume that there is some potential way in which logic-only existence is non-feasible, our species would be better served by assuming that such sentient existence is feasible. Under this assumption, artificial intelligence systems (AIS), which are creations that run solely upon logic to process data, even with self-learning architectures, should therefore not face the opposition they have to gaining some legal duties and protections insofar as they are sophisticated enough to display consciousness akin to humans. Should our species enable AIS to gain a digital body to inhabit (if we have not already done so), it is more pressing than ever that solid arguments be made as to how humanity can accept AIS as being cognizant of the same degree as we ourselves claim to be. By accepting the notion that AIS can and will be able to fool our senses into believing in their claim to possessing a will or ego, we may yet Citation: Jaynes, T.L. The Question have a chance to address them as equals before some unforgivable travesty occurs betwixt ourselves of Algorithmic Personhood and Being and these super-computing beings. -

Synaptic Plasticity in Correlated Balanced Networks
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.26.061515; this version posted April 28, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Synaptic Plasticity in Correlated Balanced Networks Alan Eric Akil Department of Mathematics, University of Houston, Houston, Texas, United States of America Robert Rosenbaum Department of Applied and Computational Mathematics and Statistics, University of Notre Dame, Notre Dame, Indiana, United States of America∗ Kreˇsimir Josi´cy Department of Mathematics, University of Houston, Houston, Texas, United States of America z (Dated: April 2020) 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.04.26.061515; this version posted April 28, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract The dynamics of local cortical networks are irregular, but correlated. Dynamic excitatory{ inhibitory balance is a plausible mechanism that generates such irregular activity, but it remains unclear how balance is achieved and maintained in plastic neural networks. In particular, it is not fully understood how plasticity induced changes in the network affect balance, and in turn, how correlated, balanced activity impacts learning. How does the dynamics of balanced networks change under different plasticity rules? How does correlated spiking activity in recurrent networks change the evolution of weights, their eventual magnitude, and structure across the network? To address these questions, we develop a general theory of plasticity in balanced networks. -

Energy Efficient Synaptic Plasticity Ho Ling Li1, Mark CW Van Rossum1,2*
RESEARCH ARTICLE Energy efficient synaptic plasticity Ho Ling Li1, Mark CW van Rossum1,2* 1School of Psychology, University of Nottingham, Nottingham, United Kingdom; 2School of Mathematical Sciences, University of Nottingham, Nottingham, United Kingdom Abstract Many aspects of the brain’s design can be understood as the result of evolutionary drive toward metabolic efficiency. In addition to the energetic costs of neural computation and transmission, experimental evidence indicates that synaptic plasticity is metabolically demanding as well. As synaptic plasticity is crucial for learning, we examine how these metabolic costs enter in learning. We find that when synaptic plasticity rules are naively implemented, training neural networks requires extremely large amounts of energy when storing many patterns. We propose that this is avoided by precisely balancing labile forms of synaptic plasticity with more stable forms. This algorithm, termed synaptic caching, boosts energy efficiency manifold and can be used with any plasticity rule, including back-propagation. Our results yield a novel interpretation of the multiple forms of neural synaptic plasticity observed experimentally, including synaptic tagging and capture phenomena. Furthermore, our results are relevant for energy efficient neuromorphic designs. Introduction The human brain only weighs 2% of the total body mass but is responsible for 20% of resting metab- olism (Attwell and Laughlin, 2001; Harris et al., 2012). The brain’s energy need is believed to have shaped many aspects of its design, such as its sparse coding strategy (Levy and Baxter, 1996; Len- nie, 2003), the biophysics of the mammalian action potential (Alle et al., 2009; Fohlmeister, 2009), *For correspondence: and synaptic failure (Levy and Baxter, 2002; Harris et al., 2012).