The Risk of Colorectal Cancer in Patients with Ulcerative Colitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inflammatory Bowel Disease Irritable Bowel Syndrome
Inflammatory Bowel Disease and Irritable Bowel Syndrome Similarities and Differences 2 www.ccfa.org IBD Help Center: 888.MY.GUT.PAIN 888.694.8872 Important Differences Between IBD and IBS Many diseases and conditions can affect the gastrointestinal (GI) tract, which is part of the digestive system and includes the esophagus, stomach, small intestine and large intestine. These diseases and conditions include inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). IBD Help Center: 888.MY.GUT.PAIN 888.694.8872 www.ccfa.org 3 Inflammatory bowel diseases are a group of inflammatory conditions in which the body’s own immune system attacks parts of the digestive system. Inflammatory Bowel Disease Inflammatory bowel diseases are a group of inflamma- Causes tory conditions in which the body’s own immune system attacks parts of the digestive system. The two most com- The exact cause of IBD remains unknown. Researchers mon inflammatory bowel diseases are Crohn’s disease believe that a combination of four factors lead to IBD: a (CD) and ulcerative colitis (UC). IBD affects as many as 1.4 genetic component, an environmental trigger, an imbal- million Americans, most of whom are diagnosed before ance of intestinal bacteria and an inappropriate reaction age 35. There is no cure for IBD but there are treatments to from the immune system. Immune cells normally protect reduce and control the symptoms of the disease. the body from infection, but in people with IBD, the immune system mistakes harmless substances in the CD and UC cause chronic inflammation of the GI tract. CD intestine for foreign substances and launches an attack, can affect any part of the GI tract, but frequently affects the resulting in inflammation. -

Nutritional Considerations in Inflammatory Bowel Disease
NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #5 Series Editor: Carol Rees Parrish, M.S., R.D., CNSD Nutritional Considerations in Inflammatory Bowel Disease by Kelly Anne Eiden, M.S., R.D., CNSD Nutrient alterations are commonplace in patients with inflammatory bowel disease. The etiology for these alterations is multifactorial. Nutrition assessment is the first step in successful nutrition management of any patient with gastrointestinal disease. Nutritional goals include assisting with nutrition risk, identifying macronutrient and micronutrient needs and implementing a nutrition plan to meet those needs. This article addresses many of the nutrition issues currently facing clinicians including: oral, enteral and parenteral nutrition, common vitamin/mineral deficiencies, medium chain triglycerides and nutrition as primary and supportive therapy. INTRODUCTION and supportive treatment in both Crohn’s and UC. The nflammatory bowel disease (IBD), encompassing following article will provide guidelines to help the both Crohn’s disease and ulcerative colitis (UC), is clinician determine nutritional risk, review specialized Ia chronic inflammatory intestinal disorder of nutrient needs and discuss nutrition as a treatment unknown etiology. A multitude of factors, including modality in the patient with IBD. drug-nutrient interactions, disease location, symp- toms, and dietary restrictions can lead to protein NUTRITION ASSESSMENT IN INFLAMMATORY energy malnutrition and specific nutritional deficien- BOWEL DISEASE cies. It is estimated that up to 85% of hospitalized IBD patients have protein energy malnutrition, based on Factors Affecting Nutritional Status abnormal anthropometric and biochemical parameters in the Patient with IBD (1,2). As Crohn’s disease can occur anywhere from There are many factors that alter nutrient intake in the mouth to anus (80% of cases in the terminal ileum), it patient with IBD. -

Chronic Viral Hepatitis in a Cohort of Inflammatory Bowel Disease
pathogens Article Chronic Viral Hepatitis in a Cohort of Inflammatory Bowel Disease Patients from Southern Italy: A Case-Control Study Giuseppe Losurdo 1,2 , Andrea Iannone 1, Antonella Contaldo 1, Michele Barone 1 , Enzo Ierardi 1 , Alfredo Di Leo 1,* and Mariabeatrice Principi 1 1 Section of Gastroenterology, Department of Emergency and Organ Transplantation, University “Aldo Moro” of Bari, 70124 Bari, Italy; [email protected] (G.L.); [email protected] (A.I.); [email protected] (A.C.); [email protected] (M.B.); [email protected] (E.I.); [email protected] (M.P.) 2 Ph.D. Course in Organs and Tissues Transplantation and Cellular Therapies, Department of Emergency and Organ Transplantation, University “Aldo Moro” of Bari, 70124 Bari, Italy * Correspondence: [email protected]; Tel.: +39-080-559-2925 Received: 14 September 2020; Accepted: 21 October 2020; Published: 23 October 2020 Abstract: We performed an epidemiologic study to assess the prevalence of chronic viral hepatitis in inflammatory bowel disease (IBD) and to detect their possible relationships. Methods: It was a single centre cohort cross-sectional study, during October 2016 and October 2017. Consecutive IBD adult patients and a control group of non-IBD subjects were recruited. All patients underwent laboratory investigations to detect chronic hepatitis B (HBV) and C (HCV) infection. Parameters of liver function, elastography and IBD features were collected. Univariate analysis was performed by Student’s t or chi-square test. Multivariate analysis was performed by binomial logistic regression and odds ratios (ORs) were calculated. We enrolled 807 IBD patients and 189 controls. Thirty-five (4.3%) had chronic viral hepatitis: 28 HCV (3.4%, versus 5.3% in controls, p = 0.24) and 7 HBV (0.9% versus 0.5% in controls, p = 0.64). -

Liver Disease in Ulcerative Colitis: an Epidemiological and Follow up Study
84 Gut 1994; 35:84-89 Liver disease in ulcerative colitis: an epidemiological and follow up study in the county of Stockholm Gut: first published as 10.1136/gut.35.1.84 on 1 January 1994. Downloaded from U Broome, H Glaumann, G Hellers, B Nilsson, J Sorstad, R Hultcrantz Abstract sclerosing cholangitis (PSC) are said to be more In an epidemiological study ofthe incidence of common in patients with UC.47 The incidence of ulcerative colitis (UC) in the county of Stock- these complications varies in different studies holm between 1955 and 1979, 1274 patients depending on selection criteria and on the with UC were discovered. Almost all these definition of hepatobiliary disease.89 The true patients had regularly been investigated with incidence of hepatobiliary dysfunction in UC, liver function tests; 142 (11%) of them showed however, can only be obtained by tests of liver signs of hepatobiliary disease. A follow up function tests such as transaminases, alkaline study on all 142 patients with abnormal liver phosphatases, and bilirubin made routinely in function and UC was made between 1989 and unselected groups of patients with UC. Because 1991 to evaluate the cause of the liver abnor- the cause of hepatic involvement in UC is mality and to find out if the liver disease had enigmatic it is important to find out if the rate affected the survival rates. At follow up, eight of this complication is increasing, because of patients were reclassified as having Crohn's factors unrelated to UC, or if it mirrors the disease, 60 had developed normal liver func- incidence ofUC in itself. -
Ulcerative Colitis Your Guide
CROHN’S & COLITIS UK SUPPORTING YOU TO MANAGE YOUR CONDITION ULCERATIVE COLITIS YOUR GUIDE 3 INTRODUCTION ABOUT THIS BOOKLET Being diagnosed with Ulcerative Colitis can be a shock, and you probably have many questions. Now that you’ve put a name to your symptoms, you can start to manage them. The more you know about your Colitis, the more you’ll be able to take an active part in decisions about your care. This booklet will give you and your family and friends a better understanding of Colitis, how it is treated, and how it will affect your life. All our publications are research-based and produced in consultation with patients, medical advisers and other health or associated professionals. They are prepared as general information and are not intended to replace specific advice from your own doctor or any other professional. Crohn’s & Colitis UK does not endorse or recommend any products mentioned. If you would like more information about the sources of evidence on which this booklet is based, or details of any conflicts of interest, or if you have any feedback on our publications, please email publications@ crohnsandcolitis.org.uk. About Crohn’s & Colitis UK We are a national charity established in 1979. Our aim is to improve life for anyone affected by Crohn’s and Colitis. We have 40,000 members and 50 Local Networks throughout the UK. Membership costs start from £15 per year with concessionary rates for anyone experiencing financial hardship or on a low income. © Crohn’s & Colitis UK 2017 & 2019 This publication is available free of charge, but we Ulcerative Colitis Edition 9d would not be able to do this without our supporters and Last review - June 2017 members. -

Fact Sheet: News from the IBD Help Center: Intestinal Complications
Fact Sheet News from the IBD Help Center INTESTINAL COMPLICATIONS The complications of Crohn’s disease and ulcerative colitis are generally classified as either local or systemic. The term “local” refers to complications involving the intestinal tract itself, while the term “systemic” (or extraintestinal) refers to complications that involve other organs or that affect the patient as a whole. Intestinal complications tend to occur when the intestinal inflammation: • is severe • extends beyond the inner lining (mucosa) of the intestines • is widespread • is chronic (of long duration) Some intestinal complications occur in both ulcerative colitis and Crohn’s disease, although they may occur more commonly in one than in the other. Not everyone will experience these complications. However, early recognition and prompt treatment are key. If you notice a change in your symptoms, be sure to contact your doctor immediately. Ulcerative Colitis-Local Complications Perforation (rupture) of the bowel. Intestinal perforation occurs when chronic inflammation and ulceration of the intestine weakens the wall to such an extent that a hole develops in the intestinal wall. This perforation is potentially life- threatening because the contents of the intestine, which contain a large number of bacteria, can spill into the abdomen and cause a serious infection called peritonitis. In colitis, this complication is generally linked with toxic megacolon (see below). In Crohn’s disease, it may occur as a result of an abscess or fistula. Fulminant colitis. This complication, which affects less than 10% of people with colitis, involves damage to the entire thickness of the intestinal wall. When severe inflammation causes the colon to become extremely dilated and swollen, a condition called ileus may develop. -

Enteric Viruses and Inflammatory Bowel Disease
viruses Review Enteric Viruses and Inflammatory Bowel Disease Georges Tarris 1,2, Alexis de Rougemont 2 , Maëva Charkaoui 3, Christophe Michiels 3, Laurent Martin 1 and Gaël Belliot 2,* 1 Department of Pathology, University Hospital of Dijon, F 21000 Dijon, France; [email protected] (G.T.); [email protected] (L.M.) 2 National Reference Centre for Gastroenteritis Viruses, Laboratory of Virology, University Hospital of Dijon, F 21000 Dijon, France; [email protected] 3 Department of Hepatogastroenterology, University Hospital of Dijon, F 21000 Dijon, France; [email protected] (M.C.); [email protected] (C.M.) * Correspondence: [email protected]; Tel.: +33-380-293-171; Fax: +33-380-293-280 Abstract: Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a multifactorial disease in which dietary, genetic, immunological, and microbial factors are at play. The role of enteric viruses in IBD remains only partially explored. To date, epidemiological studies have not fully described the role of enteric viruses in inflammatory flare-ups, especially that of human noroviruses and rotaviruses, which are the main causative agents of viral gastroenteritis. Genome-wide association studies have demonstrated the association between IBD, polymorphisms of the FUT2 and FUT3 genes (which drive the synthesis of histo-blood group antigens), and ligands for norovirus and rotavirus in the intestine. The role of autophagy in defensin-deficient Paneth cells and the perturbations of cytokine secretion in T-helper 1 and T-helper 17 inflammatory pathways following enteric virus infections have been demonstrated as well. -
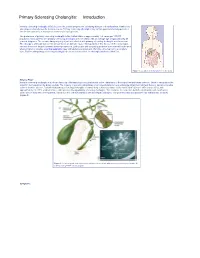
Primary Sclerosing Cholangitis: Introduction
Primary Sclerosing Cholangitis: Introduction Primary sclerosing cholangitis (PSC) is a chronic , usually progressive, stricturing disease of the biliary tree. Remissions and relapses characterize the disease course. Primary sclerosing cholangitis may remain quiescent for long periods of time in some patients; in most cases, however, it is progressive. The prevalence of primary sclerosing cholangitis in the United States is approximately 1–6 cases per 100,000 population. Most patients with primary sclerosing cholangitis are men (75%) with an average age of approximately 40 years at diagnosis. The overwhelming majority of patients affected with primary sclerosing cholangitis are Caucasian. The etiology is unknown but current opinion favors an immune cause. Management of this disease in the early stages involves the use of drugs to prevent disease progression. Endoscopic and surgical approaches are reserved for the time when symptoms develop. Liver transplantation may ultimately be required and offers the only chance for a complete cure. Patients with primary sclerosing cholangitis are at an increased risk for cholangiocarcinoma (10–15%). Figure 1. Location of the biliary tree in the body. What is PSC? Primary sclerosing cholangitis is a chronic fibrosing inflammatory process that results in the obliteration of the biliary tree and biliary cirrhosis. There is variability in the extent of involvement of the biliary system. The majority of patients with primary sclerosing cholangitis have underlying inflammatory bowel disease, namely ulcerative colitis or Crohn’s disease. Patients with primary sclerosing cholangitis are more likely to have ulcerative colitis than Crohn’s disease (85% versus 15%), with approximately 2.5–7.5% of all ulcerative colitis patients having primary sclerosing cholangitis. -

IRRITABLE BOWEL SYNDROME INFLAMMATORY BOWEL DISEASE Irritable Bowel Syndrome (IBS) Vs
IRRITABLE BOWEL SYNDROME INFLAMMATORY BOWEL DISEASE Irritable Bowel Syndrome (IBS) vs. Inflammatory Bowel Disease (IBD): IBS is a disorder of the colon characterized by symptoms of abdominal pain or discomfort associated with irregular defecation. IBD refers to a condition where a patient has either Crohn’s disease or ulcerative colitis. IBD might also be referred to as colitis, enteritis, ileitis or proctitis. IBS Symptoms: IBD Symptoms: • Abdominal pains or cramps (usually in the lower half of Crohn’s disease the abdomen) • Symptoms depend on disease location and severity. In • Excessive gas general, symptoms may include: • chronic diarrhea • feeling of a mass or • Harder or looser bowel movements than average • abdominal pain and fullness in the lower, Diarrhea, constipation, or alternating between the two tenderness (often right abdomen on the right side of • weight loss • Symptoms of IBS DO NOT include bleeding or black the lower abdomen) • fever stools Ulcerative Colitis • IBS “triggers” can include certain foods, medicines and emotional stress • Bloody diarrhea is the main symptom • IBS is not a life-threatening condition and does not make • Other symptoms can include: a person more likely to develop other colon conditions, • abdominal pain such as ulcerative colitis, Crohn’s disease or colon cancer • fever • weight loss IBS and IBD Statistics: When to See a Gastroenterologist: • One in five Americans (20% of people) have symptoms of Diagnosis: A patient usually goes through a combination of IBS, making it one of tests, including endoscopic exams, to diagnosis IBS or IBD. the most common disorders diagnosed Colon Cancer Prevention: Patients diagnosed with IBD affecting by doctors. -
Ulcerative Colitis Fact Sheet
Ulcerative Colitis What is Ulcerative Colitis? Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) that is characterized by an abnormal, prolonged immune response that creates long-lasting inflammation and ulcers (sores) in the mucosa (lining) of the large intenstine (colon), or rectum.1,2 UC and Crohn's disease both involve chronic inflammation of the intestines and classify as IBD.3 It is estimated that approximately 12.6 million people worldwide have IBD.4 Symptoms Signs and symptoms of ulcerative colitis can range from mild to severe. When the disease is active, symptoms may include:5,6 Patients with UC may experience ongoing disease symptoms, or have Fever episodes of symptom-free remission, Fatigue which can be followed by relapse or Nausea or loss flares.7 of appetite Weight loss Diarrhea Though UC is usually not a fatal (often with abdominal pain, disease, it is serious, and in some presence of blood, pus or mucus) cases, may cause life-threatening Extraintestinal manifestations (joint pain/soreness, eye complications, including an increased irritation, rash, sores risk of colorectal cancer (CRC), toxic in the mouth, etc.) megacolon/bowel obstruction and Rectal bleeding need for a colectomy.5,7 UC patients Urgent need for are almost 2.5 percent more likely to the restroom develop CRC than those without UC.8 Eect on Quality of Life Living with UC may severely aect quality of life, particularly during flares and relapses. Physical hurdles may include:9 Socio-psychological hurdles may include:9 • Pain, fatigue or discomfort -

Coexistence of Primary Biliary Cirrhosis and Inflammatory Bowel Disease Toru Shizuma* Department of Physiology, School of Medicine, Tokai University, Japan
al of urn Li o ve J r Shizuma, J Liver 2014, 3:3 Journal of Liver DOI: 10.4172/2167-0889.1000154 ISSN: 2167-0889 Mini Review Open Access Coexistence of Primary Biliary Cirrhosis and Inflammatory Bowel Disease Toru Shizuma* Department of Physiology, School of Medicine, Tokai University, Japan Abstract The coexistence of Primary Biliary Cirrhosis (PBC) and Inflammatory Bowel Disease (IBD) is uncommon, although hepatobiliary complications in IBD patients are not rare. This report reviews the English and Japanese literature and covers reported cases of concomitant PBC and IBD. We identified 2 cases of concomitant PBC and Crohn’s Disease (CD) and 18 cases of concomitant PBC and Ulcerative Colitis (UC). In most instances (15/18), IBD (CD or UC) developed before PBC, with the exception of 2 cases that were almost simultaneously diagnosed with both conditions. There is no evidence that UC cases with concomitant PBC are more severe than those without; however, the clinical features of concomitant PBC and CD are unclear due to few reports. Keywords: Primary biliary cirrhosis; Inflammatory bowel disease; such as autoimmune hepatitis or PBC [15,19,21,23]. The incidence of Crohn’s disease; Ulcerative colitis hepatobiliary diseases with UC has been reported to be 3%-15% and up to 90% with abnormal liver histology at surgery or autopsy [16,24]. Introduction PSC is best known for hepatobiliary manifestation with UC, and the Inflammatory Bowel Diseases (IBD), including Crohn’s Disease frequency of incidence of patients with concomitant PSC and IBD (CD) and Ulcerative Colitis (UC), are chronic recurrent, and has been reported to be in the range of 2.4%-7.5% [10,16,23,25,26]. -

Predicting Colorectal Cancer Occurrence in IBD
cancers Review Predicting Colorectal Cancer Occurrence in IBD Mehmet Yalchin 1,2,*, Ann-Marie Baker 2 , Trevor A. Graham 2 and Ailsa Hart 1,* 1 Inflammatory Bowel Disease Department, St. Mark’s Hospital, Watford R.d., Harrow HA1 3UJ, UK 2 Centre for Genomics and Computational Biology, Barts Cancer Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse S.q., London EC1M 6BQ, UK; [email protected] (A.-M.B.); [email protected] (T.A.G.) * Correspondence: [email protected] (M.Y.); [email protected] (A.H.) Simple Summary: Patients with inflammatory bowel disease are at an increased risk of developing colorectal cancer, and so are enrolled in a surveillance colonoscopy programme aimed at detecting and treating any signs of early cancer. This review describes the current known risk factors associated with this increased risk, explores our current molecular understanding of cancer development and reviews potential new methods (molecular and technological) designed to help the surveillance programme. Abstract: Patients with colonic inflammatory bowel disease (IBD) are at an increased risk of devel- oping colorectal cancer (CRC), and are therefore enrolled into a surveillance programme aimed at detecting dysplasia or early cancer. Current surveillance programmes are guided by clinical, endo- scopic or histological predictors of colitis-associated CRC (CA-CRC). We have seen great progress in our understanding of these predictors of disease progression, and advances in endoscopic technique and management, along with improved medical care, has been mirrored by the falling incidence of CA-CRC over the last 50 years.