CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hotels in Trichy City
MY TIRUCHI MY PRIDE Hotel Sampath & An na poorni No46C, KarurByepass Road 7094435653 Restaurant Hotel Raja Garden No28, Rockjns Road 9994447133 Guru Hotel No 13A, RoyalRoad 9840194444 HotelSrees No 6/1, LakshmiNagar 8148231333 PLARathna Residency No 4, Dindugal Main Road 7373736960 Everest Park Residency No 1, Jayalaniya 3rd Street, TVS Tolgate 9865193333 Anna Statue, Chinthamani 9894461513 Ragavendra Residency No 7/2, New KarurBye Pass Road 8012156573 Alam Residency No 3/1, Convent Road 7502478666 Maya Residency No 45, Karur Bye Pass Road 9943022000 MahalakshmiResid€n.v No 42B, Singarathope 9444546032 Rbs Residency No D17, NEE 7th Cross, Sasthri Road 7313734561 Hotel Mayas Kem Prid€ No 751, salai Road, Jayanthi Bus Stop 9626347001 Surag Residency No 2T,lllupur Road, Mannarpuram 9952645204 HotelMayas No 46, Chintamani, Karur Byepass Road 7373705712 HotelAnnamalai No 11, MC Oonalds Road 9942695999 HotelGajapria No 5&6, RoyalRoad 9842435555 Hotel Royalsathyam No 42, RoyalSathyam Building 9677756203 Kannappa Hotel Grand Gardenia No 22&25, Mannarpuram Junction 9585644000 Hotel Temple lnn No 139, ChennaiTrunk Road 7373243222 AR Hotel SBI Complex, Dindugal Main Road 9842669277 Sri Ga nd hamathi Vilas No l,AyyarHotel, Number One Tolgate 9976090118 EdgavathiHotel Santhai Gate, Samayapuram Main Road 9443189986 Hotel Meena Shankar No 21C, Melapudur Main Road 9790169197 Nellai Nadar l,less No 8/1, Collectore Office Road 9786966777 lmbala Hotel No 3/1, Convent Road 9994397865 Vamana RoyalHotel No204, East Uthira Street 9944444088 HotelSri Havagriva -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2014 [Price : Rs. 65.60 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 10F] CHENNAI, WEDNESDAY, MARCH 12, 2014 Maasi 28, Thiruvalluvar Aandu–2045 Part VI–Section 1 (Supplement) NOTIFICATIONS BY HEADS OF DEPARTMENTS, ETC. TAMIL NADU NURSES AND MIDWIVES COUNCIL, CHENNAI. (Constituted under Tamil Nadu Act III of 1926) TAMIL NADU NURSES COUNCIL ELECTORAL ROLL THE YEAR 1992 [ 1 ] DTP—VI-1 Sup. (10F)—1 2 REGISTERED NURSE FOR THE YEAR 1992 Regn.No Name of the Candidate Particulars Date of Address Regn. 31832 Selvi R. Inbam CMAI Board No.5638 31.01.1992 R.C. Street, S. Tharai MR 37969 Trained at The Salvation Kudi, Kannirajapuram Army Catherine Booth Via, Ramanad Dist. Hospital, Nagercoil. From 10.7.86 to 30.6.90. Exam: 15.6.90. 31833 Selvi P. Jeeva CMAI Board No.5624 31.01.1992 Kalady North Street, MR 37970 Trained at The Salvation Puliangudi, Army Catherine Booth Tirunelveli District Hospital, Nagercoil. 627 855. From 1.7.87 to 30.6.90. Exam: 15.6.90. 31834 Selvi J. Kamala CMAI Board No.5625 31.01.1992 Manakkarai, Puthu MR 37971 Trained at The Salvation raman Villukkuri Post, Army Catherine Booth Kanyakumari District. Hospital, Nagercoil. From 1.7.87 to 30.6.90. Exam: 15.6.90. 31835 Selvi J. Leela CMAI Board No.5626 31.01.1992 Alexanderpuram, MR 37972 Trained at The Salvation Thubacodu, Army Catherine Booth Kulasekharan Post, Hospital, Nagercoil. Kanyakumari District. From 1.7.87 to 30.6.90. -

Tamil Nadu Government Gazette Extraordinary
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2018 [Price: Rs. 44.00 Paise. TAMIL NADU GOVERNMENT GAZETTE EXTRAORDINARY PUBLISHED BY AUTHORITY No. 38] CHENNAI, MONDAY, JANUARY 29, 2018 Thai 16, Hevilambi, Thiruvalluvar Aandu–2049 Part II—Section 2 Notifi cations or Orders of interest to a section of the public issued by Secretariat Departments. NOTIFICATIONS BY GOVERNMENT LAW DEPARTMENT LIST OF NOTARIES APPOINTED AND IN PRACTICE AT THE BEGINNING OF THE YEAR 2018 (See rule 17) [G.O. Ms. No. 33, Law (Administration) Department, 29th January 2018.] No. II(2)/LAW/100(g)/2018. In exercise of the powers conferred by Section 6 of the Notaries Act, 1952 (Central Act LIII of 1952), the Governor of Tamil Nadu hereby publishes the following list of Notaries appointed by the Government of Tamil Nadu and in practice at the beginning of the year 2017 :— THE LIST OF NOTARIES Serial Name of the Notary. Residential and Professional Qualifi cations. Area in which Date of Number. Address. he is Authorised Expiry of to Practice. Certifi cate of Practice. (1) (2) (3) (4) (5) (6) CITY OF CHENNAI Thiruvalargal/Thirumathi : 1 Ghouse Ali Khan V Floor, ‘ALSA COURT’, B.A., B.L. City of Chennai 21-3-2018 72, Harrington Road, Chetpet, Chennai-600 031. First Floor, Bank of Mysore Building, Chennai-600 001. 2 F.A. Rasheed 20-A, Bawa Rawthor Road, Alwarpet, M.A., B.L. City of Chennai 8-6-2017 Chennai-600 018. and Perambalur No. 21, Sunkurama Street, District. DTP—II-2 Ex. -

India COVID-19 Hospitals and Test Centres
1) NON NETWORK HOSPITALS Sr. Name of Hospital Address City PIN No. Plot No. 3&4, 1 Narayana Health Group Sadaramangala Bangalore 560066 Industrial Area 2 NH MMI Narayana Superspeciality Hospital - Raipur Dhamtari road, lalpur Raipur 492001 A Block, Near Kela 3 Fortis Hospital Shalimar Bagh Godown, NEW DELHI, New Delhi 110088 DELHI, Mulund Goregaon Link 4 Fortis Hospitals Ltd - Mulund Road, MUMBAI, Mumbai 400078 MAHARASHTRA, On Kalyan -Shill Road, 5 Fortis Hospitals Ltd - kalyan Thane 421301 Kalyan 5 th floor, mini seashore Navi 6 Hiranandani Hospital (Fortis) road, sector-10, Vashi, 400703 Mumbai Navi Mumbai CA#8, Ideal Homes 7 SSNMC Super Speciality Hospital Bangalore 560098 Township, 8 Apollo Hospitals Sarita Vihar, NEW DELHI New Delhi 110076 Block J, Mayfield 9 CK Birla Hospital For Women-Gurgaon Gurgaon 122018 Garden, Sector 51 Mulund Goregaon Link Fortis Hospital (Telegram Channel - Fortis Mental 10 Road, MUMBAI, Mumbai 400078 Health) MAHARASHTRA, 11 Manipal Hospital 98, HAL Airport Road, Bangalore 560017 Dr Baba Saheb Bharat Ratna Dr Babasaheb Ambedkar Memorial 12 Ambedkar Road, Byculla Mumbai 400012 Hospital - Byculla East, Mumbai, M M Marg, RBI Staff 13 Jagjivan Ram Hospital - Mumbai Colony, Mumbai Mumbai 400008 Central, L M Nadkarni Marg, 14 Mumbai Port Trust Hospital - Wadala Mumbai 400037 Wadala East, Veera Desai Road, 15 Andheri Sports Complex - Mumbai Mumbai 400053 Andheri West New Mhada Colony, Municipal Capacity Building and Research (MCMCR) in 16 Savarkar Nagar, Mumbai 400076 Powai Chandivali, Powai, Taharpur Rd, Taharpur, 17 Rajeev Gandhi Super Speciality Hospital Taharpur Village, New Delhi 110093 Dilshad Garden, Tahirpur Rd, GTB 18 GTBH (Guru Teg Bahadur Hospital) Enclave, Dilshad New Delhi 110095 Garden, Jawahar Lal Nehru 19 LNH (Lok Nayak Hospital) New Delhi 110002 Marg, Central, Maulana Azad Medical College Campus, 20 LNH (MAIDS) New Delhi 110002 Bahadur Shah Zafar Marg, Metro Station, Bhagawan Mahavir 21 Dr. -

Tamil Nadu Waqf Board
GOVERNMENT OF TAMIL NADU FINAL ELECTORAL ROLL OF MUSLIM MEMBERS OF PARLIAMENT FROM TAMIL NADU 1. Thiru A. Mohammedjan - Rajya Sabha 2. Thiru K. Navaskani - Lok Sabha (Ramanathapuram Constituency) (Sd./-) (Dr.CHANDRA MOHAN. B, I.A.S.,) ELECTION AUTHORITY AND PRINCIPAL SECRETARY TO GOVERNMENT BACKWARD CLASSES, MOST BACKWARD CLASSES AND MINORITIES WELFARE DEPARTMENT, CHENNAI - 9 GOVERNMENT OF TAMIL NADU FINAL ELECTORAL ROLL OF MUSLIM MEMBERS OF STATE LEGISLATIVE ASSEMBLY OF TAMIL NADU Sl. Name of the Member Constituency No. 1. Dr. (Tmt) Nilofer Kafeel Vaniyambadi 2. Thiru M. Thamimun Ansari Nagapattinam 3. Thiru K.S. Masthan Gingee 4. Thiru T.P.M. Mohideen Khan Palayamkottai 5. Thiru K.A.M. Muhammed Abubacker Kadayanallur (Sd./-) (Dr.CHANDRA MOHAN. B, I.A.S.,) ELECTION AUTHORITY AND PRINCIPAL SECRETARY TO GOVERNMENT BACKWARD CLASSES, MOST BACKWARD CLASSES AND MINORITIES WELFARE DEPARTMENT, CHENNAI - 9 GOVERNMENT OF TAMIL NADU FINAL ELECTORAL ROLL OF EX- MUSLIM MEMBERS OF BAR COUNCIL OF TAMIL NADU 1. Thiru M.K. Khan 2. Thiru M. Syed Ismail (Sd./-) (Dr.CHANDRA MOHAN. B, I.A.S.,) ELECTION AUTHORITY AND PRINCIPAL SECRETARY TO GOVERNMENT BACKWARD CLASSES, MOST BACKWARD CLASSES AND MINORITIES WELFARE DEPARTMENT, CHENNAI - 9 ELECTION OF MEMBERS OF TAMIL NADU WAQF BOARD 2020 FINAL ELECTORAL ROLL FOR THE ELECTORAL COLLEGE OF MUTHAWALLIS OF WAQF WHOSE ANNUAL INCOME RUPEES ONE LAKH AND ABOVE 1 S. G.S. NAME OF THE WAQF NAME AND ADDRESS OF ARREARS NO. NO. INSTITUTION MUTHAWALLI / PRESIDENT / OF SECRETARY CONTRI- BUTION As on 31.03.2020 1. 2. 3. 4. 5. CHENNAI DISTRICT 1. GS.3 Ashraf Alisha & Fardalisha V.Syed Jalaludeen, Secretary, 212872 Trust, 1,St.Mary Road, 173,Kutchery Road, Mandaveli,Chennai-28 Mylapore,Chennai-4 2. -
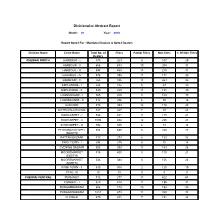
Divisionwise Abstract Report
Divisionwise Abstract Report Month: 01 Year: 2010 Report Opted For : Mandated Dealers & Opted Dealers Division Name Circle Name Total No. of Filers Partial Filers Non filers % Of Non Filers Dealers CHENNAI NORTH HARBOUR - I 375 263 5 107 29 HARBOUR - II 656 433 19 204 31 HARBOUR - III 694 463 13 218 31 HARBOUR - IV 576 394 11 171 30 HARBOUR - V 763 485 14 264 35 ESPLANADE - I 224 152 5 67 30 ESPLANADE - II 430 288 11 131 30 LOANSQUARE - I 365 228 8 129 35 LOANSQUARE - II 312 256 6 50 16 GODOWN 513 383 12 118 23 KOTHAVALCHAVADI 327 227 7 93 28 SOWCARPET - I 546 431 0 115 21 SOWCARPET - II 1096 834 12 250 23 SOWCARPET - III 592 505 4 83 14 PEDDUNAICKENPET 884 639 5 240 27 (SOUTH) RATTAN BAZAAR 413 274 6 133 32 PARK TOWN - I 394 315 9 70 18 EVENING BAZAAR 553 399 11 143 26 MOOREMARKET 515 400 5 110 21 (SOUTH) MOOREMARKET 548 384 8 156 28 (NORTH) PARK TOWN - II 630 508 2 120 19 FTAC- III 18 18 0 0 0 CHENNAI CENTRAL PERIAMET 912 277 13 622 68 EGMORE - I 620 414 11 195 31 NUNGAMBAKKAM 606 413 10 183 30 PURASAWAKKAM 1014 673 11 330 33 CHOOLAI 679 481 7 191 28 Divisionwise Abstract Report Month: 01 Year: 2010 Division Name Circle Name Total No. of Filers Partial Filers Non filers % Of Non Filers Dealers CHENNAI CENTRAL VEPERY 1434 638 26 770 54 EGMORE - II 604 416 14 174 29 AYANAVARAM 921 516 16 389 42 AMINJIKARAI 1711 944 39 728 43 PERAMBUR - I 1338 754 20 564 42 PERAMBUR - II 660 429 14 217 33 MANALI 2328 1467 34 827 36 TIRUVOTTIYUR 1156 531 16 609 53 KILPAUK 891 601 10 280 31 VADAPALANI I 1861 1259 20 582 31 VADAPALANI - II 1147 697 22 428 -

Tamil Nadu Government Gazette Extraordinary
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2021 [Price: Rs. 106.40 Paise. TAMIL NADU GOVERNMENT GAZETTE EXTRAORDINARY PUBLISHED BY AUTHORITY No. 50] CHENNAI, FRIDAY, JANUARY 29, 2021 Thai 16, Saarvari, Thiruvalluvar Aandu–2052 Part II—Section 2 Notifi cations or Orders of interest to a section of the public issued by Secretariat Departments. NOTIFICATIONS BY GOVERNMENT LAW DEPARTMENT LIST OF NOTARIES APPOINTED AND IN PRACTICE AT THE BEGINNING OF THE YEAR 2021 (See rule 17) [G.O. Ms. No. 20, Law (Administration), 29th January 2021.] No. II(2)/LAW/53(K)/2021. In exercise of the powers conferred by Section 6 of the Notaries Act, 1952 (Central Act LIII of 1952), the Governor of Tamil Nadu hereby publishes the following list of Notaries appointed by the Government of Tamil Nadu and in practice at the beginning of the year 2020 :— THE LIST OF NOTARIES Area in which he Date of Expiry Serial Name of the Notary Residential and Professional Address Qualifi cations is Authorised to of Certifi cate Number Practice of Practice (1) (2) (3) (4) (5) (6) ARIYALUR DISTRICT Thiruvalargal/Thirumathi : 1. N. Mani 9-A, PilIaiyar Koil Street, B.Sc., B.L. Perambalur and 28-9-2021 Ariyalur district. Ariyalur Districts 2. R. Natarajan 18/B2, Thopperi Street, B.A., B.L. Jayankondam 4-9-2022 Jayankondam-621 802. 3. R. Manoharan 34, Periya Aranmanai Street, B.Com., B.L. Perambalur and 15-10-2022 Ariyalur,Perambalur District. Ariyalur Districts 11, Sanjeevarayan Kovil Street, Ariyalur. 4. N. Selestin Glaster Thillai Nagar Street, Door No. -

Aqar 2009-2010
JAMAL MOHAMED COLLEGE (AUTONOMOUS) (Accredited at ‘A’ Grade by NAAC – CGPA 3.6 out of 4.0) (Affiliated to Bharathidasan University, Tiruchirappalli-620 024) Tiruchirappalli - 620 020, TAMIL NADU ANNUAL QUALITY ASSURANCE REPORT Year of Report: 2009-2010 JAMAL MOHAMED COLLEGE (Autonomous) Tiruchirappalli - 620 020, Tamil Nadu The Annual Quality Assurance Report (AQAR) of the IQAC S.No. Contents Page No 01 Part - A Plan Of Action Chalked Out By the IQAC in The Beginning 3 Of The Year Towards Quality Enhancement 02 Part - B 5 03 Part - C Details of the plans of the Institution for the next year 20 04 Any other relevant details Annexure - I Details of the Academic Association activities 21 Annexure - II Details of the New Endowments 117 Annexure - III Details of the Endowment Lectures and Various 118 programmes Annexure - IV Details of the Research Papers and Published books 124 and papers presented in Seminars / Workshops Annexure - V Details of the Initiative towards Faculty Development 258 Programme Annexure - VI Details of the Seminars /Workshops organized 262 Annexure - VII Details of the Research grants received from various 267 agencies Annexure - VIII Details of the Community Services 268 Annexure – IX Details of the Teachers and officers newly recruited 302 Annexure – X Details of the New books/journals subscribed 304 Annexure – XI Details of the Activities and support from the Alumni 313 Association Annexure – XII Details of the Achievements of the sports 326 Annexure – XIII Details of the Academic Extra Academic 331 Achievements Awards Annexure – XIV Details of the Placement services 356 JAMAL MOHAMED COLLEGE (AUTONOMOUS), TIRUCHIRAPPALLI- 620 020 INTERNAL QUALITY ASSURANCE CELL (IQAC) 01 Mr.M.J.Noordeen Sahib Presidient 02 Mr. -

Final Set PDF for Net Copy.P65
TIME TABLE - ODD SEMESTER JAMAL MOHAMED COLLEGE Day I II III IV V Order Hour Hour Hour Hour Hour ( Autonomous ) I DAY (Established in 1951) (Accredited at A Grade by NAAC - CGPA 3.6 out of 4.0) II DAY TIRUCHIRAPPALLI - 620 020. III DAY IV DAY V DAY 61st Year of Service VI DAY TIME TABLE - EVEN SEMESTER Day I II III IV V Order Hour Hour Hour Hour Hour I DAY II DAY III DAY IV DAY 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 V DAY 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456CCCALENDAR 1234567890123456789012345678901212345678901234567890123456 VI DAY 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 12345678901234567890123456789012123456789012345678901234562011 -2012 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 1234567890123456789012345678901212345678901234567890123456 -
![10E] CHENNAI, WEDNESDAY, MARCH 12, 2014 Maasi 28, Thiruvalluvar Aandu–2045](https://docslib.b-cdn.net/cover/5845/10e-chennai-wednesday-march-12-2014-maasi-28-thiruvalluvar-aandu-2045-5195845.webp)
10E] CHENNAI, WEDNESDAY, MARCH 12, 2014 Maasi 28, Thiruvalluvar Aandu–2045
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2014 [Price : Rs. 35.20 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 10E] CHENNAI, WEDNESDAY, MARCH 12, 2014 Maasi 28, Thiruvalluvar Aandu–2045 Part VI–Section 1 (Supplement) NOTIFICATIONS BY HEADS OF DEPARTMENTS, ETC. TAMIL NADU NURSES AND MIDWIVES COUNCIL, CHENNAI. (Constituted under Tamil Nadu Act III of 1926) TAMIL NADU MIDWIVES COUNCIL ELECTORAL ROLL THE YEAR 1990 [ 1 ] DTP—VI-1 Sup. (10E)—1 2 REGISTERED MIDWIFE FOR THE YEAR 1990 Regn.No Name of the Candidate Particulars Date of Address Regn. 36303 Tamil Nadu cert. Dip No: 20143 27.1.90 91. Uppu thuraipalayam, Selvi. Mariappan Malliga Govt. Rajaji Hospital , Madurai. Kolimivdi, Dhanapuram. From 1.1.86 to 30.6.86 Exam. June 86 36304 Selvi. John Chella Durai Tamil Nadu cert. Dip No: 21324 27.1.90 40B, Methu Street, Santhakumari rosemary Govt. Kasturiba Gandhi Hospital , Wordiyur, Trichy-3. Madras. From 3.1.88 to 30.6.88 Exam. June 88 36305 Selvi. Murugamali Tamil Nadu cert. Dip No: 20343 27.1.90 No.62, Thiyappa Mudali Manimegalai From 1.1.86 to 30.6.86 Exam. June Street, Madras-1. 86. Govt. R.S.R.M. Lying in Hospital, Madras-13. 36306 Selvi. Ramamudaliar Tamil Nadu cert. Dip No: 21681 27.1.90 No.12, Yerikarai Street, Mangala From 1.7.88 to 31.12.88 Exam. Dec Ranipet, N.A. Dt. 88. Govt. W & Ch. Hospital,Madras-8. 36307 Selvi. Lakshmi Narayanan Tamil Nadu cert. Dip No: 27.1.90 16.A.3, Thamman Street, Subashini 21742From 1.7.88 to 31.12.88 Muthusami Pillai Exam. -

The Tamilnadu Dr.M.G.R Medical University
Enriching Education. Empowering research. Enhancing healthcare THE TAMILNADU DR.M.G.R MEDICAL UNIVERSITY Chancellor of the University & His Excellency Governor of Tamilnadu Dr. K. Rosaiah Hon’ble Chief Minister of Tamilnadu Selvi J. Jayalalithaa Pro-Chancellor & Hon.Minister for Health Hon.Dr.V.S.Vijay Vice-Chancellor Dr. Mayil Vahanan Natarajan 4 Dr. K. Rosaiah Chancellor of the University & His Excellency Governor of Tamilnadu His Excellency Dr. K. Rosaiah, Governor of Tamil Nadu is pleased to learn that the Tamilnadu Dr. M.G.R. Medical University, Chennai is celebrating its Silver Jubilee Year on 23rd November, 2012 and is bringing out a souvenir in commemoration. The initiatives taken by the Tamilnadu Dr. M.G.R. Medical University Vice-Chancellor Dr. Mayilvahanan Natarajan and the faculty in ensuring promotion of Medical Education deserve commendation. His Excellency the Governor wishes the Silver Jubilee Year Commemoration function all success. 5 6 Hon’ble. Dr. V. S. Vijay Pro-Chancellor & Hon.Minister for Health It is pleasure to be part of the Silver Jubilee Year Celebration of this prestigious University, both as the Minister for Health and as well as the Pro- Chancellor of the University. The University has grown with great strides since its inception and today it is the second largest Medical University in India The University strives to excel in Education, Research and in providing skilled professionals to the ever expanding Health Care sector. The University has also been keen on improving the standards of Health Sciences Education I am extremely happy that the Silver Jubilee Guest House and Auditorium, which were planned for the first time in the University, and for which the foundation stones were laid by the Honorable Chief Minister of Tamil Nadu Dr. -
October – December 2017 Page No: 2/27 Bharathidasan University Quarterly Report
QUARTERLY REPORT FOR THE QUARTER ENDING 31st DECEMBER 2017 Name of the University : Bharathidasan University, Tiruchirappalli 1. Actual Enrolment: O.C. S.C. S.T. Male Female Total Male Female Total Male Female Total Allocation 10267 10733 21000 4029 4171 8200 469 331 800 Enrolment During the 0 0 0 0 0 0 0 0 0 Last Quarter Enrolment During the 0 0 0 0 0 0 0 0 0 Quarter Progressive 10267 10733 21000 4029 4171 8200 469 331 800 Total 2. Details of Special Camp Conducted: No. of NSS Volunteers Participated No. of Special O.C. S.C. S.T. Camps Conducted Male Female Total Male Female Total Male Female Total During the 13 00 572 572 00 73 73 00 05 05 Last Quarter During the 31 194 539 733 30 784 814 01 02 03 Quarter Progressive 44 194 1111 1305 30 857 887 01 07 08 Total Units 3. No. of Adopted Villages: No. of Villages No. of Villages Adopted Progressive No. of NSS Units adopted during last during the quarter Total quarter 300 0 300 300 4. Grants Position: Funds Released by State Govt. to the University: Date of Receipt of Fund: REGULAR ACTIVITIES O.C. S.C. S.T. Release Central State Central State Central State Total ( ) Total ( ) Total ( ) of Grants Share Share Share Share Share Share Grants released during 0 0 0 the Last Quarter Grants released during 4095000 1050000 105000 the Quarter Total 4095000 1050000 105000 Bharathidasan University Quarterly Report SPECIAL CAMPING PROGRAMME O.C. S.C. S.T. Receipt Central State Central State Central State Total ( ) Total ( ) Total ( ) of Grants Share Share Share Share Share Share Grants released during 0 0 0 the Last Quarter Grants released during 3685500 945000 94500 the Quarter Total 3685500 945000 94500 5.